
Evaluation of CA 125, BUN, and Creatinine Serum in Ovarian
Cancer Patients Receiving Paclitaxel-Cisplatin Chemotherapy
Treatment
Rini Noviyani
1
, P. A. Indrayathi
2
, I. N. G. Budiana
3
, Rasmaya Niruri
4
, K. Tunas
5
, Tamara Candra
Paramitha
1
1Department of Pharmacy, Faculty of Mathematics and Sciences, Udayana University, Jalan Raya Kampus UNUD, Bukit
Jimbaran Bali, Indonesia
2Department of Public Health, Faculty of Medicine, Udayana University, Jalan P.B. Sudirman, Denpasar, Indonesia
3Department of Obstetrics and Gynecology, Faculty of Medicine, Udayana University, Jalan PB. Sudirman, Denpasar,
Indonesia
4Department of Pharmacy, Faculty of Mathematics and Sciences, Sebelas Maret University, Jalan Ir. Sutami, Surakarta,
Indonesia
5Department of Public Health, Faculty of Health, Sciences, and Technology, Dhyana Pura University, Jalan Raya Padang
Luwih Tegaljaya, Kuta Utara, Indonesia
Keywords: Chemotherapy, Paclitaxel-cisplatin, Ovarian Cancer, CA 125, BUN, Creatinine Serum.
Abstract: The effectiveness and side effects of paclitaxel-cisplatin chemotherapy were assessed from the patient’s
treatment progress and toxicity level. The objective was to evaluate these two criteria through the
assessment of CA 125, BUN, and creatinine serum in patients as an approach to overcome the data
limitation at Sanglah General Hospital. Observational retrospective research with patients who had
epithelial ovarian cancer (EOC) at Stages I, II, III, and IV was conducted from February-March 2018.
Patients’ blood samples were checked before the first and after the sixth cycle of chemotherapy. The data
were processed with the Shapiro-Wilks normality test. As for the abnormally distributed data, they were
analyzed statistically using the Wilcoxon test in SPSS. The mean values of CA 125 before the first and after
the sixth chemotherapy cycles were 9,429.6 ± 1,5978.7 U/ml and 31.65 ± 36.07 U/ml, respectively (p-
value= 0109). The mean values of BUN parameter were 10.63 ± 2.95 mg/dl and 14.83 ± 7.176 mg/dl (p-
value= 0.315), respectively. The creatinine serums were averagely 0.693 ± 0.0929 mg/dl and 0.78 ± 0.2053
mg/dl (p-value= 0.417), respectively. There were differences in the levels of CA 125, BUN, and creatinine
serum before the first and after the sixth cycles.
1 INTRODUCTION
Indonesia had the third highest incidence of ovarian
cancer cases in Asia in 2012 (Raezi et al., 2016). In
2014, ovarian cancer was the second most common
gynecological cancer in women at Sanglah Hospital
(Dhitayoni and Budiana, 2017). Chemotherapy is a
therapy given to ovarian cancer patients by
administering cytotoxic drugs either in single or in
combination regimens (Braybrooke, 2011).
According to PERMENKES RI No. 72 in 2016 on
the Standards of Pharmaceutical Services in
Indonesian Hospitals, a pharmacist is obliged to
monitor the effectiveness and safety of the
chemotherapy given to the patients by preparing the
cytostatic compound and calculating the accurate
dosage according to the chemotherapy protocol
(Direktorat Jenderal Bina Kefarmasian dan Alat
Kesehatan, 2016). Paclitaxel combined with a
platinum-based regimen is known to be the most
frequently used chemotherapy regimen for epithelial
ovarian cancer (EOC) patients. The paclitaxel-
cisplatin combination is currently the first-line
regimen for ovarian cancer patients. Three trials
have established the paclitaxel-cisplatin combination
therapy as the standard regimen in advanced EOC
patients. Therefore, this regimen is used at Sanglah
Noviyani, R., Indrayathi, P., Budiana, I., Niruri, R., Tunas, K. and Paramitha, T.
Evaluation of CA 125, BUN, and Creatinine Serum in Ovarian Cancer Patients Receiving Paclitaxel-Cisplatin Chemotherapy Treatment.
DOI: 10.5220/0008238900330038
In Proceedings of the 1st Muhammadiyah International Conference on Health and Pharmaceutical Development (MICH-PhD 2018), pages 33-38
ISBN: 978-989-758-349-0
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
33

General Hospital (Ozols et al., 2000). The regimen
efficacy can be evaluated by CA 125, i.e., an ideal
tumor marker of epithelial ovarian cancer (Gupta
and Lis, 2009). Aside from its therapeutic effects,
chemotherapy triggers various side effects,
particularly on renal function. The use of paclitaxel-
cisplatin for cervix cancer at Sanglah General
Hospital has been proved to elevate BUN level and
decreases creatinine serum after the third cycle of
chemotherapy (Noviyani et al., 2016). However, the
data of the therapeutic effect and side effect of a
paclitaxel-cisplatin regimen for ovarian cancer
treatment at Sanglah General Hospital remain
limited. Hence, this research aimed to evaluate the
difference in the levels of CA 125, BUN, and
creatinine serum of EOC patients before the first
cycle and after the sixth cycle of chemotherapy.
2 MATERIALS AND METHODS
2.1 Materials
The source of the research material was patients’
medical records from January 2017 to May 2018.
All data were obtained from a collecting form and
summarized using a data summary form. This
research used a set of computer unit containing
SPSS software.
2.2 Methods
This observational retrospective research was
located at Sanglah General Hospital, Denpasar, Bali
and conducted from January to March 2018. The
research subject was selected with consecutive
sampling. The research commenced following the
approval granted by the Research and Development
Ethics Commission, Faculty of Medicine/Sanglah
General Hospital, Denpasar [Ethical Clearance
number: 87/UN.14.2/KEP/2017].
The inclusion criterion was EOC patients at
Stage I, II, III, or IV who had consented to the
collection of their CA 125, BUN, and creatinine
serum data for research purposes. The exclusion
criterion was patients with renal dysfunction before
the chemotherapy started. The information obtained
from the data collecting form was summarized and
analyzed afterward.
2.3 Data Analysis
The summarized data were analyzed statistically in
SPSS. The data were processed with the Shapiro-
Wilks normality test. The data with normal
distribution were analyzed with a dependent t-test,
while the abnormally distributed ones were later
examined using the Wilcoxon test with a 95%
confidence level (*p= 0.05). The conditions before
the first and after the sixth cycle were concluded as
significantly different if the *p-value of the collected
data was <0.05.
3 RESULTS AND DISCUSSION
3.1 Patients’ Characteristics
The number of samples collected from February to
May 2018 was three. All samples fulfilled the
inclusion criterion. Their characteristics are
described in Table 1.
Ovarian cancer risk increases sharply after the
age of 40 years old and tends to reach its peak at the
age of 50-60 years old (Arania and Indri, 2015). The
ovarian cancer risk elevates as the age increases
until around 70 years old (Goodman et al., 2003).
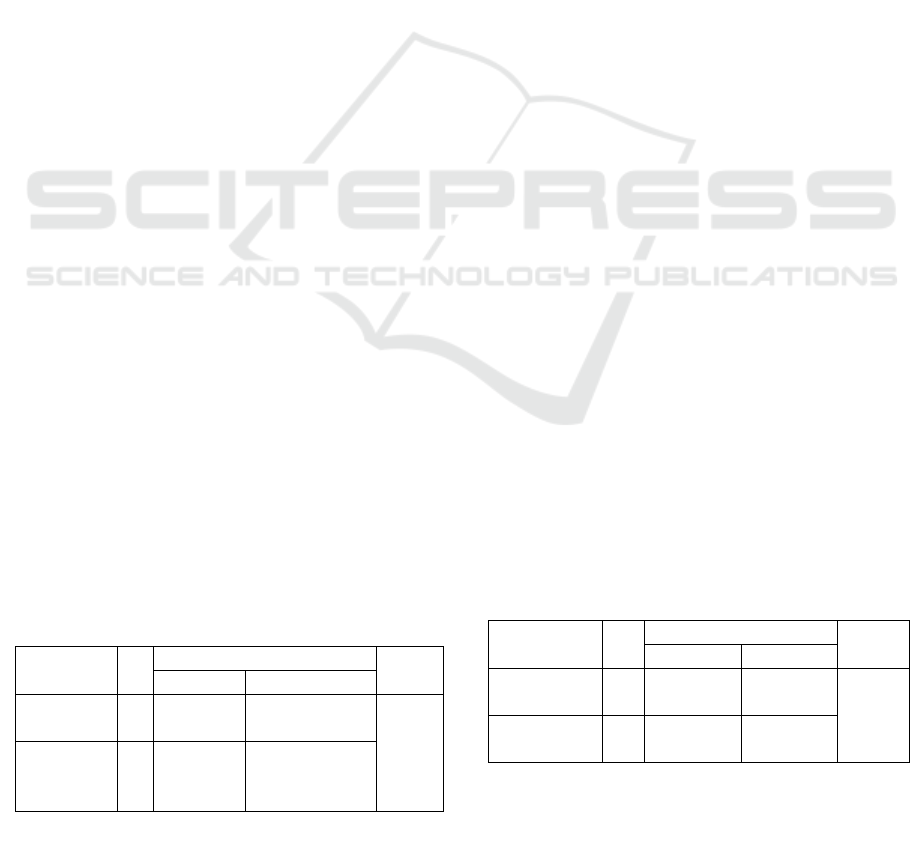
According to Table 1, the samples involved in this
research are mostly of 41-60 years old.
Women need to receive education and
information about gynecological health from the age
of adolescence. The lack of knowledge and
information about the risk factors and symptoms
leads to limited awareness of ovarian cancer. The
latest formal education of one of the three samples
involved in this research was primary school.
Meanwhile, the rest had never received any formal
education at school. The low educational level of the
Table 1: Patients’ Characteristics
Characteristics
Number
(N=3)
Percentage
(%)
Age 20-40 y.o. 1 33.3
41-60
y
.o. 2 66.7
Educational
Level
No formal
education
2 66.7
Elementary
school
1 33.3
Occupational
Status
Farme
r
1 33.3
Employee 1 33.3
Unemploy-
ed
1 33.3
Marital
Status
Married 1 33.3
Single 2 66.7
Cancer
Classification
Serous 2 66.7
Mucinous 1 33.3
Cancer Stage I 1 33.3
III 2 66.7
N
= Number of total sam
p
les
MICH-PhD 2018 - 1st Muhammadiyah International Conference on Health and Pharmaceutical Development
34
samples suggests that education and information
about ovarian cancer are highly needed since
adolescence (Rachmani et al., 2012).
One of the three samples was married and had
more than one child, while the other two were single
and had no children. Patients who have not given
birth to any children have a higher risk of
developing ovarian cancer (Permuth-Wey and
Sellers, 2009). One of the three samples was
diagnosed with Stage I ovarian cancer and the rests
were diagnosed with Stage III ovarian cancer. This
condition shows that ovarian cancer is most likely
diagnosed at later stages due to its ambiguous and
non-specific symptoms at the early stage. Patients
probably confuse them with other less severe
diseases (Roett and Evans, 2009). Also, there is
currently no routine and accurate test to detect early-
stage ovarian cancer in general population;
therefore, most women are not diagnosed until the
tumors grow and metastasize to other vital parts of
the body (Badgewell and Bast, 2007).
3.2 CA 125
CA 125 is a tumor marker used to diagnose ovarian
cancer in women. It has the highest specificity
(80%) compared to other tumor markers, such as CA
19-9 (36.4%) and CEA (8.1%) (Malati, 2007). CA
125 is secreted by abnormal epithelial cells and
found in 83% of epithelial ovarian cancer patients
(Liao et al., 2014).
In this research, CA 125 data were collected
before the first and after the sixth cycle of
chemotherapy. These data showed an abnormal
distribution. The results of the Wilcoxon test are
shown in Table 2.
According to Table 2, there is no significant
difference between the CA 125 values before the
first and after the sixth cycle of chemotherapy (p*-
value>0.05). A larger sample is, however, required
for the next stage of this research to get a more
accurate result. On the other hand, clinically, the
mean values of the samples show a decreasing CA
125 before the first and after the sixth cycle of
chemotherapy, i.e., 9,429.6 U/ml and 31.65 U/ml,
respectively The CA 125 value of a normal
individual is lower than 35 U/ml (Agarwal and
Kehoe, 2010). The reduction of CA 125 value to the
normal range after the sixth cycle suggests that the
paclitaxel-cisplatin chemotherapy contributes to
good therapeutic response in ovarian cancer patients
at Sanglah General Hospital. Based on the
information collected from 223 patients, Lee et al.
(2016) explain that the CA 125 values decrease to
the normal range after the first cycle of paclitaxel-
cisplatin chemotherapy and normalize within three
cycles of chemotherapy.
Paclitaxel works by interrupting the production
of microtubules, stabilizing the existing
microtubules, and inhibiting their destruction (Lacy
et al., 2004). It arrests the cell cycle at the G2/M
phase and leads to apoptosis (Barbuti and Chen,
2015). Meanwhile, cisplatin works by binding to
DNA―leading to the formation of interstrand and
intrastrand crosslinks―and interrupting DNA
synthesis and replication in cell proliferation (Miller
et al., 2010). The reduction of CA 125 value
compared to the previous cycle of chemotherapy
indicates good treatment response, whereas an
elevated CA 125 value signifies the possibility of
chemo-resistance, which prompts the replacement of
ongoing regimen with another therapeutic one
(Agarwal and Kehoe, 2010).
3.3 BUN
The decomposition process of protein produces a
waste product called urea. A renal function
evaluation was conducted by examining the BUN
value of the samples. An elevated BUN is a
characteristic identified in the plasma of patients
with severe renal dysfunction (Sherwood, 1996).
The BUN value increases along with the
Table 2: The Results of the Wilcoxon Test of CA
125 Before the First and After the Sixth Cycle of
Chemotherapy
N
CA 125
P
Mean SD
Before
first c
y
cle
3 9429.6 15978.74066
0.10
9 NS
After
sixth
c
y
cle
3 31.6467 36.06815
Table 3: The Results of the Dependent T-Test of
BUN Values Before the Firstnd After the Sixth
Cycle of Chemotherapy
N
BUN
P
Mean SD
Before the
first c
y
cle
3 10.6333 2.94845
0.315
NS
After the
sixth c
y
cle
3 14.8333 7.17658
N= number of samples,
SD = standard deviation,
P= significant value.
Evaluation of CA 125, BUN, and Creatinine Serum in Ovarian Cancer Patients Receiving Paclitaxel-Cisplatin Chemotherapy Treatment
35

deterioration of renal function. Therefore, the
evaluation of BUN value decides whether an ovarian
cancer patient experiences renal dysfunction (Duong
and Jin-Yew, 2006). Since the collected BUN data
before the first and after the sixth cycle of
chemotherapy showed a normal distribution, they
were analyzed with a dependent t-test. The results
are summarized in Table 3.
The kidneys receive approximately 25% of
cardiac output and have a crucial role in absorbing
drugs. The high rate of drug uptake and delivery
results in a high intracellular concentration of
substances. These substances are then processed in
an extensive metabolism that leads to the production
of reactive oxygen species and toxic metabolites
(Perazella, 2009). Another study with 18 cervical
cancer patients who undergo cisplatin chemotherapy
shows an elevated BUN value and creatinine serum
after the fifth cycle of chemotherapy (Arankumar et
al., 2012).
Paclitaxel likely leads to peripheral neuropathy
and hematological side effects, such as neutropenia
and leukopenia (Lawrenti, 2013). Meanwhile,
cisplatin tends to be dominant in causing renal side
effects. It accumulates in the kidneys and interacts
with sulfhydryl compounds, which, thereby,
increases renal membrane fragility and induces the
depletion of intracellular glutathione. Renal damage
is associated with acute focal tubular necrosis and
the dilatation of convoluted tubules and collecting
ducts. Clinically, the damage is manifested as an
increase in BUN, creatinine serum, and electrolyte
disturbance (Arankumar et al., 2012). However,
BUN values are not fully determined by patients’
renal function. The other influencing factors include
patients’ protein intake, muscle injury, necrosis, and
liver function (Duong and Jin-Yew, 2006).
3.4 Creatinine Serum
Creatinine serum is the most sensitive renal function
indicator because the human body constantly
produces this substance. Renal dysfunction causes
an increase in creatinine serum (Ignativicius and
Workman, 2006). The collected data before the first
and after the sixth cycle of chemotherapy had a
normal distributed. These data were then analyzed
with a dependent t-test. The results are shown in
Table 4.
The results showed that there was no significant
difference between the creatinine serum levels
before the first and after the sixth cycle of
chemotherapy (p> 0.05). However, clinically, there
was an elevated creatinine serum after the sixth
cycle of chemotherapy. This elevation was still
within the normal range of creatinine serum value
(0.6-1.3mg/dl) (Duong and Jin-Yew, 2006). Such
increase implies that paclitaxel-cisplatin causes a
renal side effect on patients receiving chemotherapy.
From the data of 18 patients who undergo cisplatin
chemotherapy, Arankumar et al. (2012) conclude
that the levels of the creatinine serum before the first
and after the fifth cycle of chemotherapy are
significantly different. They also state that the
creatinine serum of the patients increases by 44.87%
after the fifth cycle of the cisplatin chemotherapy
(Arankumar et al., 2012).
In addition to the effects of the chemotherapy,
several factors can contribute to the increase of
creatinine serum, such as muscular dystrophy,
malnutrition, the reduction of muscle mass, and the
use of several drugs like cimetidine and ascorbic
acid (Indrawati et al., 2011). Compared to another
platinum-based agent such as carboplatin, cisplatin
has a higher nephrotoxic effect caused by its lower
selectiveness to tumor cells. Besides, carboplatin is a
derivative of cisplatin. Therefore, carboplatin is
more stable than cisplatin; however, they exhibit an
equivalent activity against some types of cancer
(Anderson et al., 2002). To prevent any cisplatin-
induced nephrotoxicity, hydration and
supplementation are highly recommended for EOC
patients. For patients administered with ≤50 mg/m
2
cisplatin, 2-4 L NS and potassium supplementation
are required. In addition to this recommendation,
magnesium supplementation is necessary for
patients given with ≥50 mg/m
2
cisplatin. Meanwhile,
patients receiving ≥100 mg/m
2
cisplatin are
recommended to also take mannitol (Crona et al.,
2017).
Table 4: The Results of the Dependent T-Test of
Creatinine Serum Before the First and After the
Sixth Cycle of Chemotherapy
N
Creatinine Seru
m
P
Mean SD
Before the
first c
y
cle
3 0.6933 0.09292
0.417
NS
After the
sixth c
y
cle
3 0.7767 0.20526
N= number of samples,
SD = standard deviation,
P = significant value
MICH-PhD 2018 - 1st Muhammadiyah International Conference on Health and Pharmaceutical Development
36

4 CONCLUSION
There are differences in the levels of CA 125, BUN,
and creatinine serum before the first and after the
sixth cycle of chemotherapy. There are a decrease in
CA 125 and elevations in BUN level and creatinine
serum after the sixth cycle of chemotherapy.
ACKNOWLEDGMENT
The authors would like to thank the Ministry of
Research, Technology, and Higher Education of the
Republic of Indonesia, I Wayan Megadhana,
SPOG(K) as the Head of Obstetric and Gynecology
Specialist Study, and all staffs at Sanglah General
Hospital Obstetric Polyclinic, Denpasar for all of
their cooperation.
REFERENCES
Agarwal, P. and Kehoe, S. 2010. Serum tumour marker in
gynaecological cancers. J. Maturitas. 67: 46-53.
Anderson, P.O., J.E. Knoben, and W.G. Troutman. 2002.
Handbook Of Clinical Drug Data, 10th Edition. New
York: Medical Publishing Devision, McGraw-Hill.
Arankumar, P.A., G.L. Iswanatha, N. Radheshyam, H.
Mukund, and Belliyappa, M.S. 2012. Science Behind
Cisplatin – Induced Nephrotoxicity in Humans: A
Clinical Study. Asian Pac j Trop Biomed. 2(8): 640-
644.
Arania, R. and Indri W. 2015. Karakteristik Pasien Kanker
ovarium di Rumah Sakit Dr. H. Abdul Moeloek
Bandar Lampung Tahun 2009-2013. Juke Unila. Vol.
5(9).
Badgwell, D. and R.C. Bast. 2007. Early Detection of
Ovarian Cancer. J. Disease Markers. 23: 397-410.
Barbuti, A.M and Z.S. Chen. 2015. Paclitaxel Through the
Ages of Anticancer Therapy: Exploring Its Role in
Chemoresistance and Radiation Therapy: A Review. J
Cancers. 2360-2371.
Braybrooke, J. 2011. Regiment: Carboplatin + Paclitaxel.
United States of America: ASWCS Network
Chemotherapy Group.
Crona, D.J. et al. 2017. A Systematic Review of Strategic
to Prevent Cisplatin-Induced Nephrotoxicity. The
Oncologist. 22:609-619.
Dhitayoni, I.A., and I.N.G. Budiana. 2017. Profil Pasien
Kanker Ovarium di Rumah Sakit Umum Pusat
Sanglah Denpasar – Bali Periode Juli 2013 – Juni
2014. E-journal Medica. 6(3):1-9.
Direktorat Jenderal Bina Kefarmasian dan Alat Kesehatan.
2016. Peraturan Menteri Kesehatan Republik
Indonesia No. 72. Jakarta: Pemerintah Republik
Indonesia.
Duong, C.D., and L. Jin-Yew. 2006. Laboratory
Monitoring in Oncology. Journal of Oncology
Pharmacy Practice. 12(4):22-25.
Goodman, M.T., Howe, H.L., Tung, K.H., Hotels, J.,
Miller, B.A., and Coughlin, S.S. 2003. The Incidence
of Ovarian Cancer by Race and Ethnicity in the United
States, 1992–1997. American Cancer Society. Vol.
97(10): 2676-2685.
Gupta, D., and Lis, C.G. 2009. Role of CA 125 in
PredictingOvarian Cancer Survival – A Review of the
Epidimiological Literature. Journal of Ovarian
Cancer. 2(13): 1-20.
Ignatavicius and Workman. 2006. Medical Surgical
Nursing Critical Thinking for Collaborative Care.
Vol. 2. Elsevier Sauders: Ohia.
Indrawati, S. et al. 2011. Pedoman Interpretasi Data
Klinik. Jakarta: Kementrian Kesehatan Republik
Indonguptaesia.
Lacy, F.C, Armstrong L.L., Goldman P.M., and Lance
L.L. 2004. Drug Information Handbook 12
th
Edition.
United States: Lexi-Comp Incorporated.
Lawrenti, H. 2013. Kemoterapi Golongan Taxane. CDK-
209. vol. 40(10):790-794.
Lee, M., et al. 2016. Clinical Significance of CA125 Level
after the First Cycle of Chemotherapy on Survival of
Patients with Advanced Ovarian Cancer. J Yonsei
Med. 57(3): 580-587.
Liao, X.Y., G.J. Huang, C. Gao, G.H. Wang. 2014. A
Meta-analysis of Serum Cancer Antigen 125 Array for
Diagnosis of Ovarian Cancer in Chinese. Journal of
Cancer Research and Therapeutics. 10(2):221-224.
Malati, T. 2007. Tumor Markers: An Overview. Indian
Journal of Clinical Biochemistry. 22(2):17-31.
Miller, R.P., K. R.K. Tadagavadi, G. Ramesh, and W.B.
Reeves. 2010. Mechanisms of Cisplatin
Nephrotoxicity.
Noviyani, R., P.A. Indrayani, H. Thabrany, Andrijono,
N.G. Budiana. 2016. Differences in the Value of
Blood Urea Nitrogen and Creatinine Serum in
Cervical Cancer Squamous Cell Stadium IIB-IIIB
before and after Chemotherapy Paclitaxel-Cisplatin for
Six Cycles in Sanglah General Hospital Denpasar,
Bali. Asian J Pharm Clin Res. 10(2): 381-384. Ozols,
R.F., et al. 2000. Epithelial Ovarian Cancer.
Philadelphia: Lippincott Williams and Wilkins. 981-
1057.
Perazella, M.A. 2009. Renal Vulnerability to Drug
Toxicity. Clin J Am Soc Nephrol. 4: 1275-1283.
Permuth-Wey and Sellers. 2009. Methods of Molecular
Biology, Cancer Epidemiology. Epidemiology of
Ovarian Cancer. 472:413-417.
Raezi, S. et al. 2016. The Incidence and Mortality of
Ovarian Cancer and their Relationship with the
Human Development Index in Asia. E-cancer Medical
Science. 10(628).
http://ecancer.org/journal/10/full/628-the-incidence-
and-mortality-of-ovarian-cancer-and-their-
relationship-with-the-human-development-index-in-
asia.php. Accessed on 2
nd
March 2018.
Evaluation of CA 125, BUN, and Creatinine Serum in Ovarian Cancer Patients Receiving Paclitaxel-Cisplatin Chemotherapy Treatment
37

Rachmani, Berlian, S. Zahroh, C. Kusyogo. 2012. Sikap
Remaja Perempuan Terhadap Pencegahan Kanker
Serviks Melalui Vaksinasi HPV di Kota Semarang.
Media Kesehatan Masyarakat Indomesia. Vol. 11(1);
34-38.
Roett, M.A. and P. Evans. 2009. Ovarian Cancer: Review.
Am Pham Physician. 80(6): 609-616.
Sherwood, L. 1996. Fisiologi Manusia, Edisi II, Jakarta:
Penerbit Kedokteran EGC.
MICH-PhD 2018 - 1st Muhammadiyah International Conference on Health and Pharmaceutical Development
38
