
The Preservation of Liposomes During Air Drying Using a Matrix
Containing Maltodextrin and HPMC
Raditya Weka Nugraheni
1, 2*
, Helmy Yusuf
1
, Dwi Setyawan
1
1
Department of Pharmaceutics, Airlangga University, Jalan Dharmawangsa Dalam, Surabaya, Indonesia
2
Faculty of Health Sciences, University of Muhammadiyah Malang, Jalan Bendungan Sutami, Malang, Indonesia
Keywords: Liposomes, Air drying, Maltodextrin, HPMC, DDA
Abstract: This study used maltodextrin as a protectant to stabilize liposomes during air drying and Hydroxypropyl
Methyl Cellulose (HPMC) as solid dispersion matrix that could provide a barrier to the coalescence of
liposomes. The purpose was to optimize the composition of the matrix to protect liposomes. The liposome
suspension was prepared with the thin-film hydration method using three lipid components with the molar
ratio of SPC:DDA:Chol = 9:3:1. The maltodextrin was dissolved in water and used in the experiment as the
hydration liquid. The formulations included maltodextrin and HPMC with 4 (four) different ratios. Then,
they were air-dried at the same condition (40ºC for 120 hours). The solid products were characterized using
Powder X-Ray Diffraction (PXRD), Differential Scanning Calorimetry (DSC), and Scanning Electron
Microscopy (SEM). The PXRD analysis showed that all of the formulations developed in this study had an
amorphous structure. However, the formulations showed peak splitting in the DSC analysis. The differences
in the crystalline lamellar thickness of maltodextrin might be the cause of these results during the air drying.
The successful preservation of liposomes was analyzed using SEM photomicrography. Compared with the
other formulations, F2MO3 created the best protection for liposomes. The inclusion of HPMC as a
dispersion matrix into the liposome formulation potentially inhibits crystal formation during the drying
process and, therefore, provides better protection for the lipid bilayer.
1 INTRODUCTION
Liposome has particular advantages in vaccine
delivery, especially when cationic lipid components
are included in its formulation as a lipid bilayer
(Agger et al., 2008). One of the most potential
cationic lipids is DDA (dimethyl dioctadecyl
ammonium bromide) (Kaur et al., 2014). The use of
DDA in liposome formulations is still complicated
because it involves stability issues. First, due to its
physical instability, DDA is not suitably used as a
single lipid constituent (Kett et al., 2015). Second,
the electrostatic repulsion caused by its positive
charge is not sufficient to prevent the physical
aggregations of liposomes (Kallerup et al., 2015).
This research offers strategies to enhance the
stability of liposomes by adding sugars and
dispersing matrix, i.e., hydroxypropyl
methylcellulose (HPMC), into the developed
formulation. The role of sugar, e.g., maltodextrin,
and HPMC is to provide the glassy amorphous
matrix to inhibit the recrystallization of the
components in the formulations (Corveleyn &
Remon, 1996; Ingvarsson et al., 2011; Yu, 2001). To
prove the hypothesis, this research evaluated the
physical behavior of these components during the
drying process. Air drying is preferable because it is
simpler (i.e., it does not need special equipment) and
cheaper compared with the alternatives, namely
freeze-drying and spray-drying process.
2 MATERIALS AND METHOD
2.1 Materials
For the components of the liposomes, this research
used Dimethyl-dioctadecyl ammonium (Sigma
Aldrich, Singapore) and soy phosphatidylcholine S-
Nugraheni, R., Yusuf, H. and Setyawan, D.
The Preservation of Liposomes During Air Drying Using a Matrix Containing Maltodextrin and HPMC.
DOI: 10.5220/0008238600150018
In Proceedings of the 1st Muhammadiyah International Conference on Health and Pharmaceutical Development (MICH-PhD 2018), pages 15-18
ISBN: 978-989-758-349-0
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
15
100 (Lipoid GmBh, Germany). The addition of
cholesterol (Sigma-Aldrich, Singapore) to the
formulations was expected to provide a membrane
stabilizer. Maltodextrin (Sigma-Aldrich, E:13-17)
was used as a lyoprotectant to stabilize the
liposomes during the air-drying process. HPMC
15000 (Metolose 90SH-15000SR, Shin-Etsu, Japan)
functioned as a dispersion matrix to increase the
mass of the end product. This research chose
methanol (analytical grade, Merck) as a solvent to
facilitate the mixing of liposomal ingredients.
2.2 Methods
The liposomes were prepared with the thin-film
hydration methods. The membranes consisted of
SPC, DDA, and cholesterol dissolved in methanol.
The molar ratio of the constitutive elements of the
lipid bilayer was SPC:DDA:Cholesterol = 9:3:1. The
lipid phase was then evaporated in a vacuum
condition at 45°C for 60 minutes using a rotary
evaporator (Büchi, Germany). The process left a thin
film on the evaporator wall. Afterward, the
hydration procedure started using the pre-warmed
solution of maltodextrin (5 mL) in various
concentrations (Table 1). The hydration process was
carried out at a temperature of 50°C for 10 minutes.
The appearance of white-milky suspension indicated
a successful formation of liposomes. Then, the
liposome suspensions were sonicated for 5 minut es.
The HPMC powder was weighed according to the
details in Table 1 and dispersed in 5 mL of purified
water to form HPMC gel. The liposome suspensions
were added to the HPMC gel, mixed until
homogeneous, and divided into the vials for drying.
The characterizations of the solid products by
XRD, DSC, and SEM were carried out according to
Nugraheni et al. (2017). The condition of the XRD
analysis was as follows: Cu as an anode, Kα filter, a
generator set to 40 kV/30 mA, and room
temperature. The study was carried out at a 2theta
Table 1: The formulations of liposomes
Formulation Codes Maltodextrin
Concentrations
HPMC Concentrations Maltodextrin:HPMC
(Weight ratio)
F2MO1 5% 2.5% 2:1
F2MO2 10% 2.5% 4:1
F2MO3 5% 7.5% 2:3
F2MO4 10% 7.5% 4:3
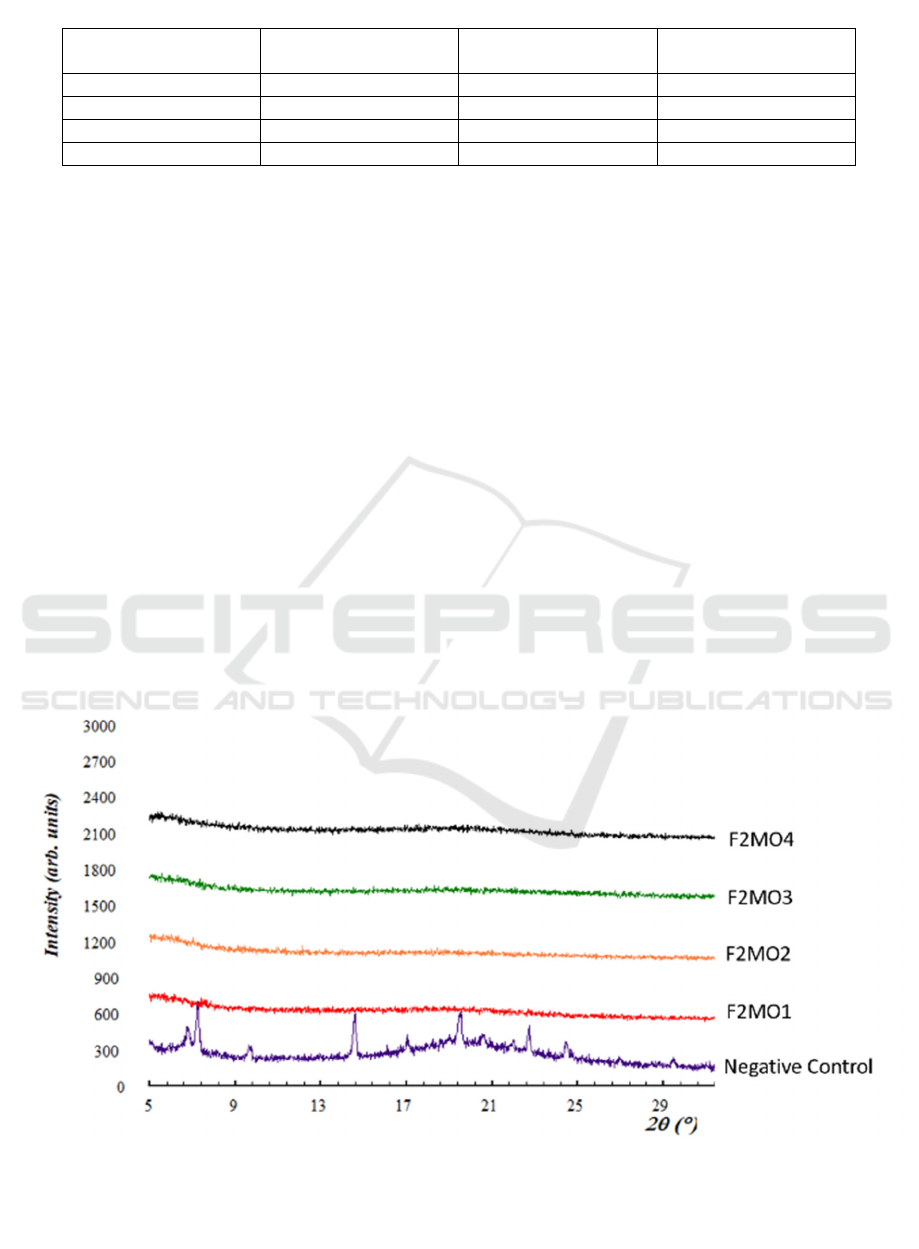
Figure 1: The X-Ray Diffraction pattern of samples containing different concentrations of maltodextrin and
HPMC (as seen in Table 1). The negative control (purple) was dried lipid components without maltodextrin
and HPMC.
MICH-PhD 2018 - 1st Muhammadiyah International Conference on Health and Pharmaceutical Development
16
range of 5 to 40°. Meanwhile, in the DSC thermal
analysis, the samples were placed in aluminum
crucibles and scanned from 30°C-300°C with a
heating rate of 10°C/min. The morphology of the
liposomes in the solid gel was analyzed with SEM.
The portions of the dried product were scattered and
glued onto 25-mm diameter plates, which were
attached to the SEM specimen mounts. The
specimens were sputter-coated with a 5-nm layer of
Au-Palladium.
3 RESULTS AND DISCUSSION
The solid systems of all formulations were relatively
amorphous. Compared with the negative control,
there was nearly no high-intensity peak detected in
the X-Ray diffractogram pattern analysis (Figure 1).
The amorphous system is the ideal condition
because it can preserve the liposomes in the
formulations. This physical feature is necessary
because the crystalline components can damage the
integrity of the bilayer membrane and cause ruptures
on it (Li et al., 2016). The X-Ray diffractogram of
all formulations also strongly confirmed the role of
vitrification mechanism to preserve the liposomes
during drying (Ingvarsson et al., 2011).
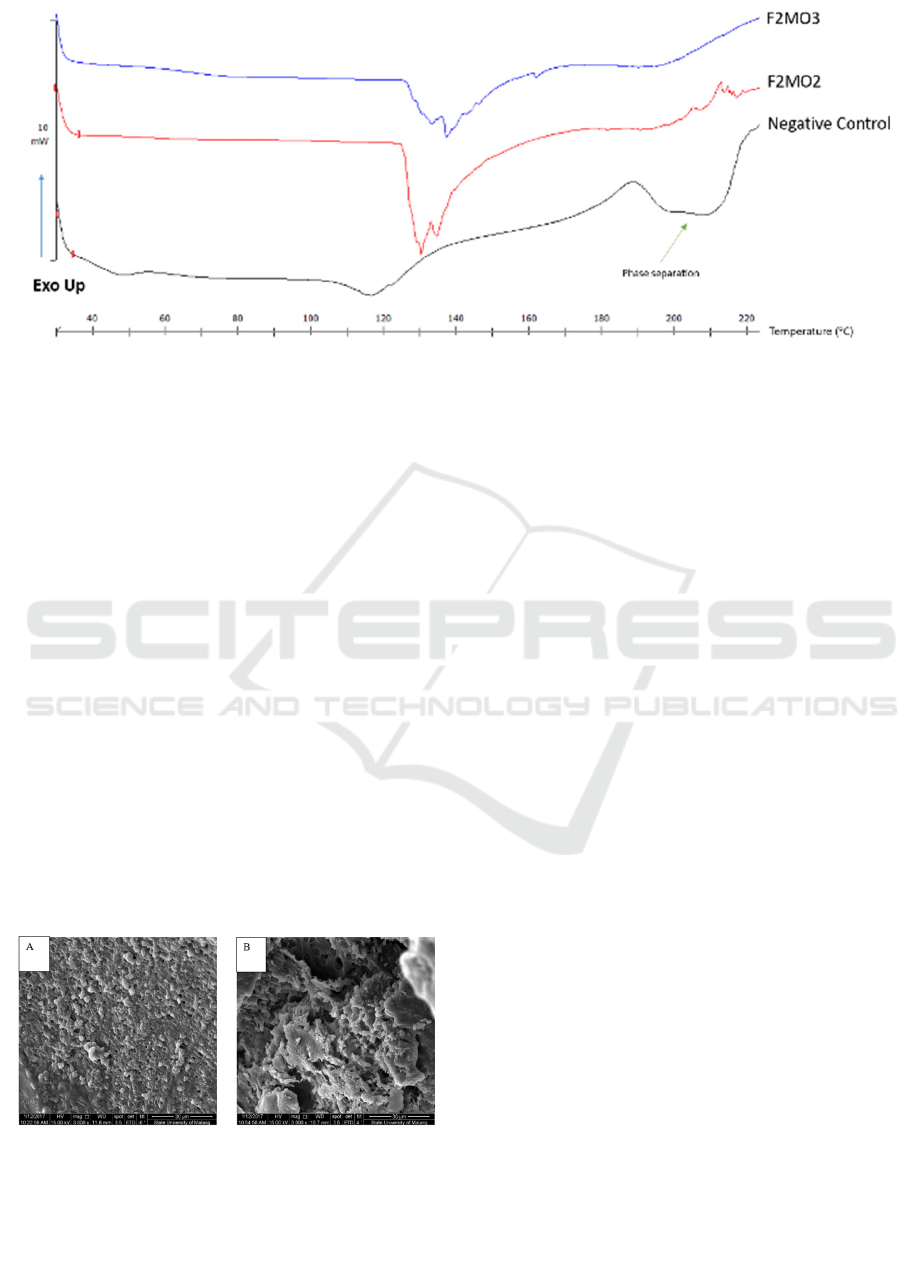
Contrary to Nugraheni et al. (2017) in which the
liposome formulations from maltodextrin were
freeze-dried, the DSC profiles in this research
exhibited endothermic peak splitting at 120-150⁰C
(Figure 2). These results imply the heterogeneity of
the samples. The different melting points of the
crystal with a different lamellar thickness in the
polymer were probably the cause of the endothermic
peak splitting (Montanheiro et al., 2016). The
reorganization or recrystallization of amorphous
material in thermal treatment, e.g., heating during
the air-drying process, might be responsible for this
phenomenon (Pereira et al., 2016).
Compared with the other formulations, F2MO3
and F2MO2 were more homogeneous (Figure 2).
The phase separation for samples containing neither
Maltodextrin nor HPMC occurred at 120-180⁰C.
This finding shows that both components are crucial
in the formulations.
The SEM photograph (Figure 3) of the sample’s
surface changed when the maltodextrin increased.
The increase of maltodextrin in the formulations
containing the same amount of HPMC produced
more porous and rougher surface. The porosity of
the matrix is essential to facilitate the rehydration of
the samples (Yusuf et al., 2017). F2MO3 produced a
Figure 2: The DSC thermogram pattern of samples containing different concentrations of maltodextrin and
HPMC (as seen in Table 1). The negative control (black) was dried lipid components without maltodextrin and
HPMC.
Figure 3: The different SEM microphotographs,
indicating that the increase of maltodextrin in
formulations containing the same amount of
HPMC produces a different surface profile. (A)
F2MO3, (B) F2MO4.
The Preservation of Liposomes During Air Drying Using a Matrix Containing Maltodextrin and HPMC
17

smoother surface than F2MO4, which would
accommodate the preservation of liposomes in the
matrix.
Yusuf et al. (2017) have created several
formulations with different disaccharides, but the
resultant crystallinity profile was unfavorable
because the phase separations still occurred in the
results. However, this research developed the
formulations using amorphous oligosaccharide. The
finding shows that F2MO3 is the formulation that
meets the desired physical characteristics. The high
HPMC-maltodextrin ratio inhibits molecular re-
arrangement and, thereby, provides better protection
for the liposome vesicles.
4 CONCLUSIONS
The inclusion of Maltodextrin and HPMC as a
dispersion matrix into the liposome formulations
potentially inhibits crystal formation during the
drying process and, consequently, provides better
protection for the lipid bilayer in liposomes.
REFERENCES
Agger, E.M., Rosenkrands, I., Hansen, J., Brahimi, K.,
Vandahl, B.S., Aagaard, C., Werninghaus, K.,
Kirschning, C., Lang, R., Christensen, D., Theisen,
M., Follmann, F., & Andersen, P., 2008. Cationic
Liposomes Formulated with Synthetic Mycobacterial
Cord factor (CAF01): A Versatile Adjuvant for
Vaccines with Different Immunological Requirements.
PLOS ONE 3, e3116.
https://doi.org/10.1371/journal.pone.0003116
Corveleyn, S., & Remon, J.P., 1996. Maltodextrins as
lyoprotectants in the lyophilization of a model protein,
LDH. Pharm. Res. 13, 146–150.
Ingvarsson, P.T., Yang, M., Nielsen, H.M., Rantanen, J.,
& Foged, C., 2011. Stabilization of liposomes during
drying. Expert Opinion on Drug Delivery 8, 375–388.
https://doi.org/10.1517/17425247.2011.553219
Kallerup, R.S., Madsen, C.M., Schiøth, M.L., Franzyk, H.,
Rose, F., Christensen, D., Korsholm, K.S., & Foged,
C., 2015. Influence of trehalose 6,6′-diester (TDX)
chain length on the physicochemical and
immunopotentiating properties of DDA/TDX
liposomes. European Journal of Pharmaceutics and
Biopharmaceutics 90, 80–89.
https://doi.org/10.1016/j.ejpb.2014.10.015
Kaur, R., Henriksen-Lacey, M., Wilkhu, J., Devitt, A.,
Christensen, D., & Perrie, Y., 2014. Effect of
Incorporating Cholesterol into DDA: TDB Liposomal
Adjuvants on Bilayer Properties, Biodistribution, and
Immune Responses. Molecular Pharmaceutics 11,
197–207. https://doi.org/10.1021/mp400372j
Kett, V., Yusuf, H., McCarthy, H., & Chen, K.H., 2015.
Liposomal delivery system. US Patent Document
Li, J., Hu, M., Xu, H., Yu, X., Ye, F., Wang, K., Luan, X.,
Li, L., & Zhang, D., 2016. Influence of type and
proportion of lyoprotectants on lyophilized
ginsenoside Rg3 liposomes: Lyophilized ginsenoside
Rg3 liposomes. Journal of Pharmacy and
Pharmacology 68, 1–13.
https://doi.org/10.1111/jphp.12489
Montanheiro, T.L. do A., Passador, F.R., Oliveira, M.P.
de, Durán, N., Lemes, A.P., Montanheiro, T.L. do A.,
Passador, F.R., Oliveira, M.P. de, Durán, N., &
Lemes, A.P., 2016. Preparation and Characterization
of Maleic Anhydride Grafted Poly (Hydroxybutyrate-
CO-Hydroxyvalerate) – PHBV-g-MA. Materials
Research 19, 229–235. https://doi.org/10.1590/1980-
5373-MR-2015-0496
Nugraheni, R.W., Setyawan, D., & Yusuf, H., 2017.
Physical Characteristics of Liposomal Formulation
Dispersed in HPMC Matrix and Freeze-Dried Using
Maltodextrin and Mannitol as Lyoprotectant.
Pharmaceutical Sciences, 23, 285–292.
https://doi.org/10.15171/PS.2017.42
Pereira, G.C., Rzatki, F.D., Mazzaferro, L., Forin, D.M.,
Barra, G.M. de O., Pereira, G.C., Rzatki, F.D.,
Mazzaferro, L., Forin, D.M., & Barra, G.M. de O.,
2016. Mechanical and Thermo-Physical Properties of
Short Glass Fiber Reinforced Polybutylene
Terephthalate upon Aging in Lubricant/Refrigerant
Mixture. Materials Research 19, 1310–1318.
https://doi.org/10.1590/1980-5373-mr-2016-0339
Yu, L., 2001. Amorphous pharmaceutical solids:
preparation, characterization and stabilization.
Advanced Drug Delivery Reviews 48, 27–42.
https://doi.org/10.1016/S0169-409X(01)00098-9
Yusuf, H., Nugraheni, R., Mulyadi, N.A., Setyawan, D., &
Rosita, N., 2017. Phase behavior of dried–DDA
liposomal formulation dispersed in HPMC matrix in
the presence of saccharides. Int J PharmTech Res 10,
50–56.
MICH-PhD 2018 - 1st Muhammadiyah International Conference on Health and Pharmaceutical Development
18
