
Effect of Acute Administration of Eel (Anguilla bicolor bicolor) Oil to
Hematological Parameters in Mice
Heru Sasongko
Department of Pharmacy, Faculty of Mathematics and Natural Sciences, Universitas Sebelas Maret. Jl. Ir. Sutami 36A
Surakarta 57126, Central Java Indonesia
Keywords: acute toxicity, eel, fish oil, hematology, omega-3
Abstract: Eel (Anguilla bicolor bicolor) is a fish consumed as food and found in Indonesia. The eel oil is known to
contain fatty acids like omega-3. The study aims to determine the effect of acute administration of eel oil to
hematological parameters in mice. A total of 24 male Swiss Webster mice were divided into four groups.
Group I as the control was not administered by eel oil and administered aquadest instead, group II, III, and
IV were given 0.09; 0.25 and 0.74 g/ 20 g B.W, respectively, of eel oil. The oil was administered for 14
days. On day 14
th
, blood sample was taken from orbital sinus. The hematological parameter consisted of the
amount of erythrocyte, leukocyte, hemoglobin, hematocrit, lymphocyte, neutrophil and platelet levels were
measured. The data were statistically analyzed by one way ANOVA followed by LSD test. The result is
acute administration of eel oil at dose 0.74 g/20 g B.W has caused significant change of hematological
parameters, except for erythrocytes and platelets level. The conclusion of this study was that at a dose of
0.74 g/20 g B.W eel oil had an effect on blood hematologic changes in mice.
1 INTRODUCTION
Anguilla bicolor bicolor is a type of fish consumed
in many countries like Japan, China, Germany, and
France (Sasongko et al., 2017). This fish has not
been used optimally in Indonesia because many
people do not know about it and it is more expensive
than other types of fish. Eel oil is reported to contain
fatty acids such as eicosapentaenoic acid (EPA) and
docosahexaenoic acid (DHA) (Baeza et al., 2014;
Kusharto et al., 2014). Eicosapentaenoic acid and
docosahexaenoic acids are part of Omega-3
polyunsaturated fatty acids (Amissi et al., 2016).
Fish contains nutrients such as proteins, fatty acids,
minerals and vitamins (Vitamins A, B3, B6, B12, E,
and D) and it is good for health (Suleria et al.,
2015).
Fish oil is known as the source of
polyunsaturated fatty oil and widely used for the
pharmaceutical purpose and food supplement
(Daiello et al., 2015; Suleria et al., 2015). Fatty
acids have been used as baby food by some health
agents. In previous study, eel oil was shown to have
an effect of decreasing total cholesterol tested on
animal models (Sasongko et al., 2017). Omega-3
polyunsaturated fatty acids such as EPA and DHA
have been shown to protect the cardiovascular
system, to protect the body from cancer,
inflammatory and autoimmune diseases (Amissi et
al., 2016; Simopoulos, 2002).
Despite the various benefit of eel oil, there is no
research about the safety of eel oil consumption.
Toxic effects of the eel oil might occur. This study
aimed to investigate the toxic effect of acute
administration of eel oil to the hematological
parameters in male mice.
2 MATERIALS AND METHOD
2.1 Materials
Eel (Anguilla bicolor bicolor) aging between 3-4
month and weighing 100-200 gram were collected
from UNAGI business department, Universitas
Sebelas Maret. Studies were carried out using male
Swiss Webster mice (20 – 30 g). Mice were obtained
from the Faculty of Medicine Universitas Sebelas
Maret, Surakarta, Indonesia. All animal handling
procedures have been approved by the ethics
Sasongko, H.
Effect of Acute Administration of Eel (Anguilla bicolor bicolor) Oil to Hematological Parameters in Mice.
DOI: 10.5220/0008238500110014
In Proceedings of the 1st Muhammadiyah International Conference on Health and Pharmaceutical Development (MICH-PhD 2018), pages 11-14
ISBN: 978-989-758-349-0
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
11
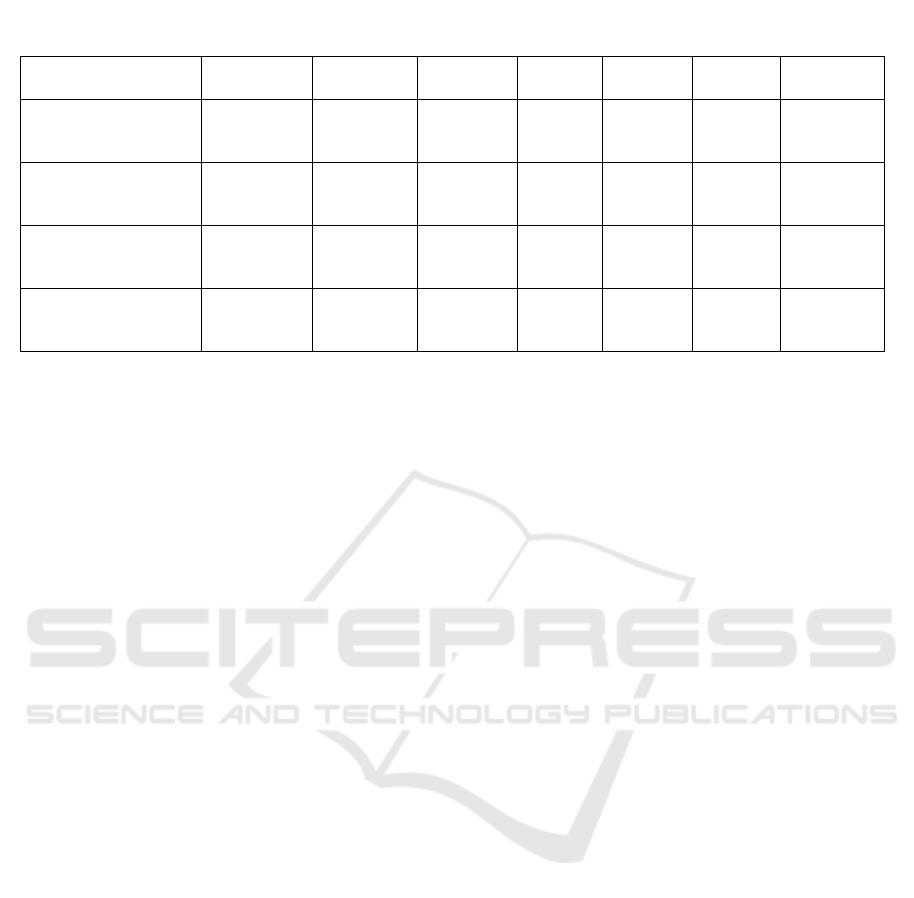
Table 1: Hematological parameters following 14 days observation exposure of eel oil in mice.
Groups
WBC
(10
4
xµL)
RBC
(10
4
xµL)
HGB
(g/
d
L)
HCT
(%)
PLT
(10
4
xµL)
LYM
(10
2
xµL)
NEU
(10
2
xµL)
Negative control
81.83 ±
17.30
779.17 ±
87.89
12.62 ±
1.42
38.15 ±
4.37
69.13 ±
24.67
85.33 ±
4.55
14.67 ±
4.55
0.09 g/20 gB.W.
55.66 ±
20.72
665.17 ±
117.42
10.05 ±
1.68
34.42 ±
4.08
69.82 ±
13.76
66.80 ±
13.67
16.53 ±
13.67
0.25 g/20 gB.W.
66.33 ±
19.19
789.00 ±
160.74
12.40 ±
2.59
40.92 ±
8.93
59.03 ±
31.62
82.18 ±
10.40
16.82 ±
10.40
0.74 g/20 gB.W.
54.16 ±
15.06*
873.33 ±
88.37
14.53 ±
1.82*
43.13 ±
3.94*
81.07 ±
34.36
59.33 ±
29.32*
26.90 ±
5.09*
Symbols represent statistical significance. *p < 0.05, as compared to negative control group. n = 6 animals in each
group. WBC: White blood cell; RBC: Red blood cell; HGB: Hemoglobin; HCT: Hematocrit; PLT: Platelet; LYM:
Lymphocyte; NEU: Neutrophile.
committee of the Faculty of Medicine, Universitas
Sebelas Maret with the number 231/II/HREC/2018.
2.2 Methods
2.2.1 Extraction
The method of eel extraction followed the method
undertaken by Sasongko et al., (2017).
2.2.2 Animal Experimental Design
The research using 24 male mice were divided into
four groups consisting of 6 mice per group. Group I
as control was given aquadest, group II, III, and IV
were given 0.09; 0.25, and 0.74 g/20 g B.W,
respectively, of eel oil. The oil was administered for
14 days. On day 14
th
, blood sample was taken from
orbital sinus. Blood sample were collected to
determine hematological parameters.
2.2.3 Measurement of Haematological
Parameters
Hematological parameters measurement including
the amount of erythrocyte (RBC), leukocyte (WBC),
hemoglobin (HGB), hematocrit (HCT), lymphocyte
(LYM), neutrophil (NEU) and platelet levels (PLT).
Measurement of hematologic parameters followed
by Guder et al. (2014); Pusterla and Higgins (2017).
2.3 Data Analysis
The data were statistically analyzed by one way
ANOVA followed by LSD test.
3 RESULT AND DISCUSSION
The administration of eel oil for 14 days did not
show mortality in test animals. The analysis of blood
parameters can be used for diagnosing the organs or
tissues function disorder. The hematological
examination may describe the function of body
organs and physiological status (Bachri et al., 2017).
It is relevant to risk evaluation as changes in the
hematological system have a higher predictive value
for human toxicity (Rodeiro et al., 2018). The result
of hematological can be seen in Table 1.
3.1 The Number of Leukocytes (WBC)
Leukocytes is involved in protecting the body as
immune system cells against infectious agents or
foreign invaders (Bachri et al., 2017). The white
blood cells along with neutrophils and lymphocytes
as their derivatives can prevent pathogens invasion
or disease-causing. The microorganisms such as
bacteria and viruses through phagocytosis process,
identifying and destroying dangerous or cancerous
cells (Walters et al., 1989).
The results shows that at dose of 0.74 g/20 gB.W
eel oil have a significant effect of decreased levels of
leukocytes (p <0.05). This is probably related to the
immunomodulatory effect on lipid-mediator
generation in leukocytes from omega‐3 fatty acids
from eel oil (Morlion et al., 1996; Vedin et al.,
2008).
MICH-PhD 2018 - 1st Muhammadiyah International Conference on Health and Pharmaceutical Development
12

3.2 The Number of Erythrocytes
(RBC)
The red blood cells travel in blood carrying
hemoglobin in the circulation. Their main function is
carrying waste carbon dioxide back to the lungs and
distributing oxygen to body tissues (Bachri et al.,
2017; Snyder and Sheafor, 1999). The results shows
that at dose of 0.74 g/20 gB.W eel oil is not
significant with negative control (p <0.05).
3.3 The Number of Hemoglobin (HGB)
Hemoglobin (HGB) is the protein contained in red
blood cells that is responsible for delivery of oxygen
to the tissues. To ensure adequate tissue
oxygenation, a sufficient hemoglobin level must be
maintained (Billett, 1990). The results shows that at
dose of 0.74 g/20 gB.W eel oil have a significant
effect on increased levels of hemoglobin (p <0.05).
These results suggest that fatty acids from eel oil
share the property of gamma-globin gene
inducibility (Liakopoulou et al., 1995). Gamma-
globin to mediate high-level expression of
hemoglobin (Arcasoy et al., 1997; Pestina et al.,
2009).
3.4 The Number of Hematocrits (HCT)
The hematocrit measures the volume of red blood
cells compared to the total blood volume (red blood
cells and plasma) (Billett, 1990). The results shows
that at dose of 0.74 g/20 gB.W eel oil have a
significant effect on increased levels of hematocrit
(p <0.05). This is consistent with an increase in
amount of red blood cells from each dose of eel oil
although statistically not significantly different.
3.5 The Number of Platelet Levels
(PLT)
Platelets is the blood cells that help the body in the
blood clotting process to stop bleeding or process
coagulation via interactions with vessel endothelial
(Garraud and Cognasse, 2015). The results showed
that at dose of 0.74 g/20 gB.W eel oil had not a
significant difference with negative control (p
<0.05).
3.6 The Number of Lymphocytes
(LYM)
A lymphocyte is a type of white blood cell that is
part of the immune system. There are two main
types of lymphocytes: B cells and T cells. The B
cells produce antibodies that are used to attack
invading bacteria, viruses, and toxins (Pubmed,
2018). The results showed that at dose of 0.74 g/20
gB.W eel oil had a significant effect on decreasing
the levels of lymphocyte (p <0.05). This is
consistent with the decreased amount of white blood
cells from each dose of eel oil although statistically
not significantly different. This may be due to the
effect of omega-3 fatty acid supplementation on
cytokine production and lymphocyte proliferation
(Meydani et al., 1991).
3.7 The Number of Neutrophils (NEU)
Neutrophils is the most commonly found in immune
cells of human blood. These cells form a defence
after a person got an infection (Hayashi et al., 2003).
The results shows that at dose of 0.74 g/20 gB.W eel
oil have a significant effect of increased levels of
neutrophil (p <0.05). Fatty acid omega-3 dan omega-
6 differentially influence the plasma free fatty acid
profile with impact on neutrophil functions. Lipid-
based parenteral nutrition may thus exert a profound
influence on sequelae and status of
immunocompetence and inflammation (Mayer et al.,
2003).
4 CONCLUSIONS
The acute administration of eel oil can cause the
change of hematological parameters in the dose 0.74
g/20 g b.w, except for erythrocytes and platelets
level. There is significant difference on
hematological parameters of mice that treated with
eel oil compared to the control group (p < 0.05).
ACKNOWLEDGEMENTS
The author would like to thank Universitas Sebelas
Maret for funding this research with the Hibah
Fundamental PNBP Grants scheme.
REFERENCES
Amissi, S., Rasul Niazi, Z., Burban, M., Kessler, R.,
Canuet, M., Toti, F., Monassier, L., Boehm, N.,
Auger, C., Meziani, F., Schini-Kerth, V.B., 2016.
0369 : The optimized omega-3 EPA: DHA 6:1 product
prevents the monocrotaline-induced pulmonary
Effect of Acute Administration of Eel (Anguilla bicolor bicolor) Oil to Hematological Parameters in Mice
13

arterial hypertension and vascular remodeling in rats.
Arch. Cardiovasc. Dis. Suppl., Printemps de la
Cardiologie : Recherche Fondamentale et Clinique -
Centre de Congrès Dijon.7-8 April 2016 8, 244.
Arcasoy, M.O., Romana, M., Fabry, M.E., Skarpidi, E.,
Nagel, R.L., Forget, B.G., 1997. High levels of human
gamma-globin gene expression in adult mice carrying
a transgene of the deletion-type hereditary persistence
of fetal hemoglobin. Mol. Cell. Biol. 17, 2076–2089.
Bachri, M.S., Yuliani, S., Sari, A.K., 2017. Effect of
subchronic administration of nutmeg (Myristica
fragrans Houtt) ethanolic extract to hematological
parameters in rat. IOP Conf. Ser. Mater. Sci. Eng. 259,
12009.
Baeza, R., Mazzeo, I., Vílchez, M.C., Gallego, V.,
Peñaranda, D.S., Pérez, L., Asturiano, J.F., 2014.
Effect of thermal regime on fatty acid dynamics in
male European eels (Anguilla anguilla) during
hormonally-induced spermatogenesis. Aquaculture
430, 86–97.
Billett, H.H., 1990. Hemoglobin and Hematocrit, in
Walker, H.K., Hall, W.D., Hurst, J.W. (Eds.), Clinical
Methods: The History, Physical, and Laboratory
Examinations. Butterworths, Boston.
Daiello, L.A., Gongvatana, A., Dunsiger, S., Cohen, R.A.,
Ott, B.R., 2015. Association of fish oil supplement use
with preservation of brain volume and cognitive
function. Alzheimers Dement. 11, 226–235.
Garraud, O., Cognasse, F., 2015. Are Platelets Cells? And
if Yes, are They Immune Cells? Front. Immunol. 6.
Guder, W.G., Narayanan, S., Wisser, H., Zawta, B., 2014.
Diagnostic Samples: From the Patient to the
Laboratory: The Impact of Preanalytical Variables on
the Quality of Laboratory Results. John Wiley & Sons.
Hayashi, F., Means, T.K., Luster, A.D., 2003. Toll-like
receptors stimulate human neutrophil function. Blood
102, 2660–2669.
Kusharto, C.M., Widyasari, R.A.H.E., Budywiryawan,
Wiyono, E.S., Sugengherisuseno, 2014. Nutritive
Value and Fatty Acids Profile of Fresh Indonesian Eel
(Anguilla bicolor) and Kabayaki. J. Sains Kesihat.
Malays. Malays. J. Health Sci. 12.
Liakopoulou, E., Blau, C.A., Li, Q., Josephson, B., Wolf,
J.A., Fournarakis, B., Raisys, V., Dover, G.,
Papayannopoulou, T., Stamatoyannopoulos, G., 1995.
Stimulation of fetal hemoglobin production by short
chain fatty acids. Blood 86, 3227–3235.
Mayer, K., Fegbeutel, C., Hattar, K., Sibelius, U., Krämer,
H.-J., Heuer, K.-U., Temmesfeld-Wollbrück, B.,
Gokorsch, S., Grimminger, F., Seeger, W., 2003. ω-3
vs. ω-6 lipid emulsions exert differential influence on
neutrophils in septic shock patients: impact on plasma
fatty acids and lipid mediator generation. Intensive
Care Med. 29, 1472–1481.
Meydani, S.N., Endres, S., Woods, M.M., Goldin, B.R.,
Soo, C., Morrill-Labrode, A., Dinarello, C.A.,
Gorbach, S.L., 1991. Oral (n-3) Fatty Acid
Supplementation Suppresses Cytokine Production and
Lymphocyte Proliferation: Comparison between
Young and Older Women. J. Nutr. 121, 547–555.
Morlion, B.J., Torwesten, E., Lessire, H., Sturm, G.,
Peskar, B.M., Fürst, P., Puchstein, C., 1996. The effect
of parenteral fish oil on leukocyte membrane fatty acid
composition and leukotriene-synthesizing capacity in
patients with postoperative trauma. Metab. - Clin. Exp.
45, 1208–1213.
Pestina, T.I., Hargrove, P.W., Jay, D., Gray, J.T., Boyd,
K.M., Persons, D.A., 2009. Correction of Murine
Sickle Cell Disease Using γ-Globin Lentiviral Vectors
to Mediate High-level Expression of Fetal
Hemoglobin. Mol. Ther. 17, 245–252.
Pubmed, 2018. Lymphocytes - National Library of
Medicine. PubMed Health. URL
https://www.ncbi.nlm.nih.gov/pubmedhealth/PMHT00
22042/ (accessed 7.20.18).
Pusterla, N., Higgins, J., 2017. Interpretation of Equine
Laboratory Diagnostics. John Wiley & Sons.
Rodeiro, I., Remirez, D., Flores, D., 2018. Sacha Inchi
(Plukenetia volubilis L.) powder: acute toxicity, 90
days oral toxicity study and micronucleus assay in
rodents. J. Pharm. Pharmacogn. Res. 6, 17–26.
Sasongko, H., Efendi, N.R., Budihardjo, A., Farida, Y.,
Amartiwi, T., Rahmawati, A.A., Wicaksono, A.,
Sugiyarto, 2017. Solvent and extraction methods
effects on the quality of eel (Anguilla bicolor) oil. J.
Phys. Conf. Ser. 795, 12021.
Sasongko, H., Sugiyarto, S., Budiharjo, A., Efendi, N.R.,
2017. Anti-hypercholesterolemia effects and quality of
eel (Anguilla bicolor) oil. Int. J. Sci. Appl. Sci. Conf.
Ser. 2, 174–180.
Simopoulos, A.P., 2002. Omega-3 Fatty Acids in
Inflammation and Autoimmune Diseases. J. Am. Coll.
Nutr. 21, 495–505.
Snyder, G.K., Sheafor, B.A., 1999. Red Blood Cells:
Centerpiece in the Evolution of the Vertebrate
Circulatory System. Integr. Comp. Biol. 39, 189–198.
Suleria, H.A.R., Osborne, S., Masci, P., Gobe, G., 2015.
Marine-Based Nutraceuticals: An Innovative Trend in
the Food and Supplement Industries. Mar. Drugs 13,
6336–6351.
Vedin, I., Cederholm, T., Freund Levi, Y., Basun, H.,
Garlind, A., Faxén Irving, G., Jönhagen, M.E.,
Vessby, B., Wahlund, L.-O., Palmblad, J., 2008.
Effects of docosahexaenoic acid–rich n−3 fatty acid
supplementation on cytokine release from blood
mononuclear leukocytes: the OmegAD study. Am. J.
Clin. Nutr. 87, 1616–1622.
Walters, M.D.S., Matthei, I.U., Kay, R., Dillon, M.J.,
Barratt, T.M., 1989. The polymorphonuclear leucocyte
count in childhood haemolytic uraemic syndrome.
Pediatr. Nephrol. 3, 130–134.
MICH-PhD 2018 - 1st Muhammadiyah International Conference on Health and Pharmaceutical Development
14
