
Adsorption of Pb (II) from Aqueous Solutions by Pectic Acid
Microspheres
Fen Li
1
, Jianjun Li
1,3
, Xiaoyan Wen
2
, Xiaoyong Li
1
, Yanhong Bai
1
,
Yun Yang
1
and Zhao Xu
1*
1
Department of Chemistry, Xi’an Jiaotong University, Xi’an 710061, China
2
Xi’an Modern Chemistry Research Institute, Xi’an 710065, China
3
Key Laboratory of Resource Biology and Biotechnology in Western China (Northwest University), Ministry of Education,
School of Life Science, Northwest University, Xi’an 710069, China
Keywords: Adsorption, Pectic Acid, Pb (II), Microspheres
Abstract: Pectin or modified pectin is used to remove the heavy metal ions in aqueous solution. The adsorption ability
of pectin microspheres (PMs) and pectic acid microspheres (PAMs) for Pb (II) in aqueous solution were
characterized by the parameters such as pH, initial concentration, and contact time for Pb (II) removal in this
work. The results showed that adsorption for 150 min at pH 5 was the optimal condition. The maximum
adsorption capacity of PMs and PAMs for Pb (II) was 127 mg·g
-1
and 325 mg·g
-1
, respectively. Five-cycle
reusability tests demonstrated microspheres could be repeatedly used. All the results confirmed that PAMs
which presented outstanding adsorption capability and reusability could be a good candidate for wastewater
purification.
1 INTRODUCTUION
Heavy metal pollution has drawn much attention
because of its high toxicity and nonbiodegrad ability
(Jorgetto et al., 2015). The heavy metal accumulated
in water would affect people’s health through various
ways to a certain extent. Researches showed that
excessive heavy metal ions which could damage the
human brain and nervous system are intangible risk
for human beings (Chojnacka, 2010). For these
reasons, it has always been an urgent task for
researchers engaged on environmental security to
seek more reasonable ways of treating water pollution.
The mainly methods of removing heavy metals
are chemical precipitation (España et al., 2006),
adsorption (Shariful et al., 2017), membrane filtration
(Mortaheb et al., 2010), ion-exchange (Fonseca et al.,
2005), electrodialysis (Mohammadi et al., 2005), and
so on. Compared with conventional methods,
bioadsorption (Chen et al., 2017) is recently
considered as the most advisable method for heavy
metal removal due to its efficiency, reproducibility,
and environmental friendliness. According to reports
(Celus et al., 2017), pectin is regarded as a suitable
candidate among the available bioadsorbents. In this
study, pectin and modified pectin were used to
remove the heavy metal in an aqueous solution.
Pectin substances belonging to the group of
natural biopolymers are the ionic plant
polysaccharides (Liu et al., 2003). Their capacity in
aqueous solutions was proved in numerous studies
mainly due to their unique properties such as
hydrophilicity, biodegradability, nontoxicity
(Serguschenko et al., 2007). But pectin had a low
adsorption capacity when directly used to remove
heavy metal ions because esterified residues were not
active. Thus, in our work pectin was modified to
improve the adsorption ability.
In this study, the work aims at investigating the
different adsorption ability of pectin, and pectic acid
(PA) which was prepared from pectin by pH-
modification. Batches of experiments were
performed to evaluate its adsorption capacities for Pb
(II) in either single or binary metal ion solutions at
various pH values, contact time, and initial
concentrations. The results showed that the
adsorption ability of PAMs is higher than that of PMs
and their high adsorption performance would provide
great potential for water treatment. The highlight of
this research is that PAMs was efficient in removing
Pb (II) with 325 mg·g
-1
from aqueous solutions at pH
5; PAMs that have absorbed Pb (II) are readily
removed from aqueous solutions and can be reused;
Li, F., Li, J., Wen, X., Li, X., Bai, Y., Yang, Y. and Xu, Z.
Adsorption of Pb (II) from Aqueous Solutions by Pectic Acid Microspheres.
DOI: 10.5220/0008189203010305
In The Second International Conference on Mater ials Chemistry and Environmental Protection (MEEP 2018), pages 301-305
ISBN: 978-989-758-360-5
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
301

high selective for Pb (II) of PAMs was showed in
mixed aqueous solutions of Cd (II) and Pb (II).
2 METHODS AND MATERIALS
2.1 Materials
Citrus pectin (CAS: 9000-69-5) was purchased from
Sigma Biotechnology Co. (America). Pb(NO
3
)
2
,
CaCl
2
and CdCl
2
·2.5H
2
O were got from Xi’an
Chemical Reagent Factory. All other reagents were of
analytical grade. The water used to prepare the
solutions was deionized water.
2.2 Preparation of PA and PAMs
2.2.1 Preparation of PA
The PA was prepared by method as described (Ilse et
al., 2009). Briefly, 10g citrus pectin power was
dissolved in 500 ml 1.0 mol·L
-1
NaOH ethanol
solution on a magnetic stirrer for 5 h at 4 ℃. Placed
overnight, the solution was filtered and the residue
was dissolved into 50% ethanol (v/v) adding 3 mol·L
-
1
HCl until the pH=1.5. After 1.5 h, the solution was
filtered and the residue was dissolved in 250 ml 50%
ethanol (v/v) containing 1% HCl stirring at 25 ℃ for
0.5 h. The mixture was filtered and the resultant
composites were washed with 50% ethanol (v/v) with
three times. After freeze-dried, the PA was obtained.
The esterification degree of pectin and PA were
determined using titrimetric method (Afanas’Ev et al.,
1984) and the results were 47.90% and 0.90%,
respectively.
2.2.2 Preparation of PAMs and PMs
The PA solution was prepared by dissolving 0.3 g of
PA power in 10 mL of deionized water (3%, w/v).
Using syringe (1 mL, 0.45 #) added the pectin
solution to the calcium chloride solution (5%, w/v)
dropwise, the needle was about 5 cm from the liquid
level. After 20 min, PAMs were collected through a
membrane filter separation, and then washed
thoroughly by deionized water. PMs were prepared
using the same procedure as PAMs, excepting the
concentration of calcium chloride solution was used
as 10%.
2.3 Characterization of PA and PAMs
FTIR (Fourier Transform infrared) spectra of PA,
pectin and microspheres were obtained by an FTIR
spectrometer (Nicolet AVATAR 360, Thermo
Instrument Company, Madison, USA) at the wave
number range of 400-4000 cm
−1
. SEM (TM-1000
SEM, Hitachi, Japan) was used to observe the surface
microstructure and morphology of PAMs and PMs.
2.4 Adsorption Experiments of Pb (II)
2.4.1 Adsorption Experiments
The experiments were performed at pH (1, 2, 3, 4, 5,
6), contact time (10, 20, 30, 60, 90, 120, 150, 180,
210, and 240 min) and initial concentration of Pb (II)
(50, 100, 150, 200, 250, 300, 350, 400, 450, 500, 600,
700 mg·L
−1
). To test the selective adsorption, a total
of 50 mg of microspheres was added into 100 mL
mixed solution which contains Cd (II) and Pb (II)
with the initial concentration of 250 mg·L
−1
at initial
pH 5.0. The reusability of microspheres was
evaluated via sequential cycles of adsorption-
desorption in a binary ion mixture system of Cd (II)
and Pb (II). After adsorption for Cd (II) and Pb (II),
the microspheres were collected, washed with
deionized water and constantly stirred for 1 h in
0.002M Na
2
EDTA solution for desorption. Then, the
microspheres were filtered and washed thoroughly
until Cd (II) and Pb (II) concentration in the filtrate
was almost equal to zero. The collected microspheres
were further used in the next adsorption cycle. The
regeneration tests were conducted for five times
under the same conditions to evaluate the reusability
of microspheres.
2.4.2 Calculation of Adsorption Capacity
The concentration of metal ions was measured by a
flame atomic absorption spectrophotometer (AA1700,
FULI Instrument, China). The adsorption capacity (q
e
)
was calculated by the equation:
m
VCC
q
e
e
)(
0
(1)
Where q
e
(mg·g
−1
) is the adsorption capacity, C
0
(mg·L
−1
) and C
e
(mg·L
−1
) are respectively initial and
final concentrations of metal ions, respectively. V (L)
is the volume of the metal ion solution, and m (g) is
the mass of the dried adsorbent.
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
302
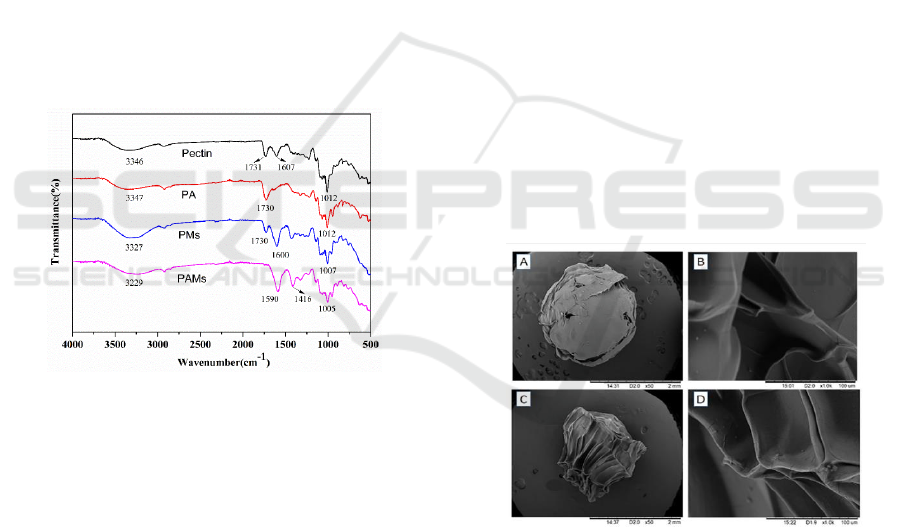
3 RESULTS AND DISSCUSION
3.1 Characterization of PA and PAMs
3.1.1 FTIR Analysis
To investigate the chemical structure of PA and PMs,
the results of FTIR are shown in Figure 1. The main
characteristic absorption bands of pectin were
summarized as follows: 3346 cm
-1
(-OH group), 1731
cm
-1
(-C=O in free carboxylic acid groups), 1607
cm
−1
(-C=O in non-free carboxyl group ) (Martinez et
al., 2012). The peak at 1607 cm
-1
of PA dispeared
demonstrating that the non-free carbonyl group in PA
decreased, and the peak of 1730 cm
-1
increased,
indicating that the free carboxyl group increased. For
PMs, the intensity at 1730 cm
-1
and 3327 cm
-1
became
weaker, compared with pectin spectrum. For PAMs
spectrum, new bands at 1416 cm
-1
appeared and 3229
cm
-1
dropped off compared with PA spectrum,
indicating that pectin and PA had a cross-linking
reaction successfully with Ca (II).
Figure 1: FTIR spectra of pectin, PA, PMs, and PAMs.
3.1.2 SEM Observation
The images of PMs and PAMs at different
magnification were recorded via SEM in Figure 2, by
which the surface morphology and texture of each
sample was mapped out. The surface of PMs with
fewer folds exhibits some small cracks and there are
more uniform folds on the PAMs surface. The mean
diameters of two microspheres all were 2 mm and had
an integral surface, which could facilitate the
separation and recycling of samples.
3.2 Effect of pH on Adsorption
The initial pH of aqueous solutions is an important
parameter that greatly influences the adsorption
property of an adsorbent. The experiments on effect
of pH were performed at initial pH 1.0-6.0 in whcih
Pb (II) solution with initial concentration of 130
mg·L
−1
and adsorption time lasted 240 min. The
results are displayed in Figure 3. At pH 1.0, both of
PMs and PAMs have the lower adsorption rate; when
the pH increased from 2.0 to 6.0, the adsorption rate
of PMs increased while that of PAMs tends to a
plateau. At pH 6.0, the adsorption rate of PMs and
PAMs for Pb (II) respectively reached maximum
values, about 69% and 95%, respectively. That may
be because –COO groups on microspheres donated
their electron pairs to Pb (II) to form complex, when
the pH was low, the carboxyl group in pectin was
protonated and the complexation of Pb (II) with the
active group was reduced. The degree of protonation
decreased with the pH rising, and the number of
active group participating in the complexation
increased. There are more carboxyl groups binding
site with Pb (II) in PA compared with pectin, so the
adsorption capacity of PAMs is higher than PMs’s.
When the value of pH in the solution reached to 6.0,
Pb (II) have tended to precipitate. There would be less
amount of Pb (II) remained consequently showing a
high adsorption rate in the result. The initial pH of the
Pb (II) solution was close to pH 5.0, while the
adsorption rate of the microspheres at pH 4 was
almost equal to that of them at pH 5, so the
optimization of pH in equilibrium adsorption is 5.0.
Figure 2: SEM images of PMs (A×50 and B×1000) and
PAMs (C×50 and D×1000).
3.3 Effect of Contact Time on
Adsorption
The experiments on influence of contact time were
conducted with initial Pb (II) concentration of 130
mg·L
−1
at pH 5.0. Figure 4 shows the effect of contact
time on Pb (II) adsorption of two kinds of
microspheres. After 90 min, the amount of adsorption
of PMs and PAMs on Pb (II) were 74% and 96%,
Adsorption of Pb (II) from Aqueous Solutions by Pectic Acid Microspheres
303
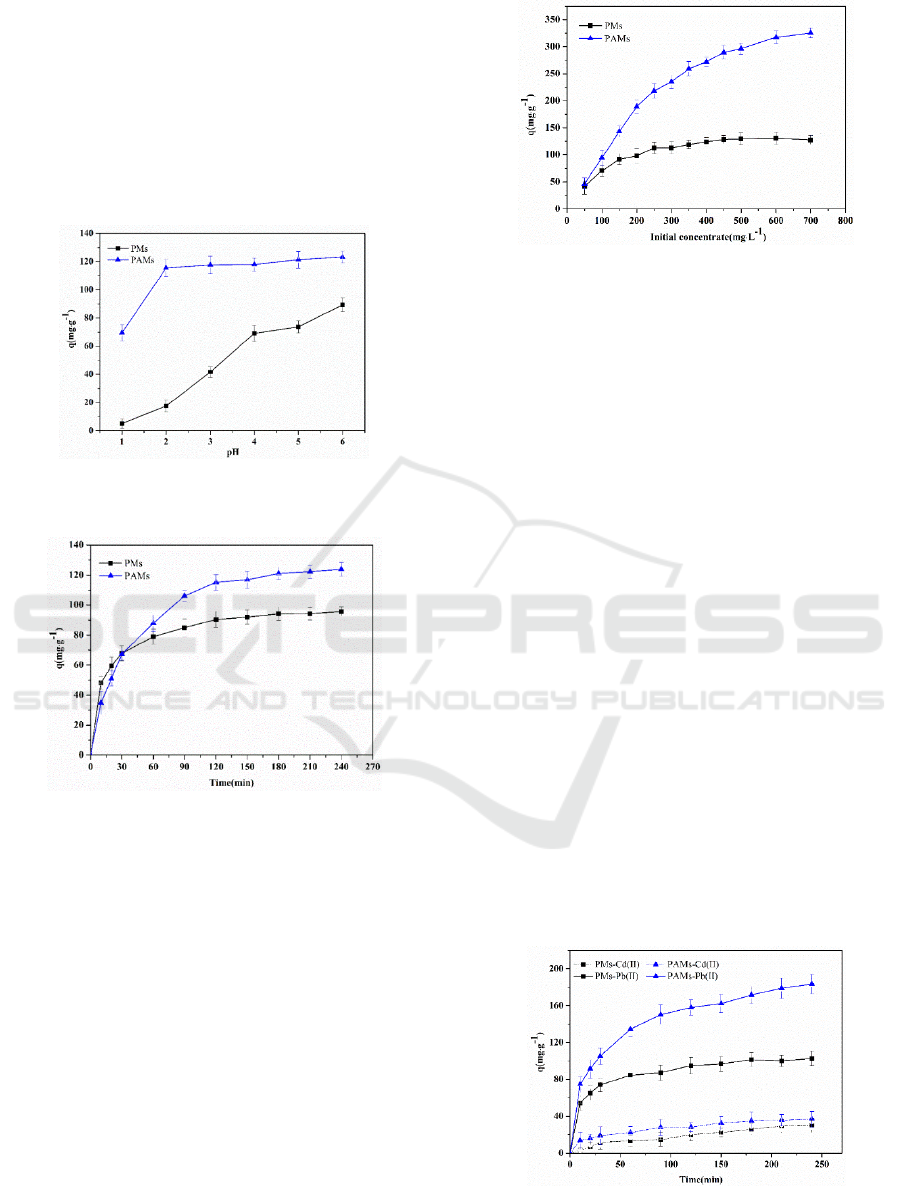
respectively. Then, the adsorption capacities
increased slowly with the increase of contact time
until reaching adsorption equilibrium at 150 min. It
was a slow process in which the metal ion diffusion
into pores and the adsorption by interior surface while
almost all facial adsorption sites of microspheres
have been occupied. The maximum adsorption
capacity of PMs and PAMs was 95.74 mg·g
-1
and
123.84 mg·g
-1
at pH 5.0.
Figure 3. Effects of pH on adsorption.
Figure 4: Effects of contact time on adsorption.
3.4 Effect of Initial Concentration on
Adsorption
The experiments on impact of initial Pb (II)
concentration were carried out at pH 5.0 for 150 min.
The effects of the initial Pb (II) concentration on the
adsorption of the microspheres are shown in Figure 5.
For PAMs, the amount of the adsorbed ions increased
slowly until approached the plateau at C
0
= 600
mg·L
−1
. The maximum adsorption capacity of PAMs
were 325 mg·g
−1
. Obviously, it was about 2.5 times
as much as that of PMs (127 mg·g
−1
), which indicated
the ability of chelating with metal ions of PAMs was
significantly improved by modified within the
experimental.
Figure 5: Effects of initial Pb (II) concentration on
adsorption.
3.5 Selective Adsorption
The tests of selective adsorption were carried out in
a binary ion mixture system of Cd (II) and Pb (II) at
pH 5.0 and with different contact time. The results
of the selective adsorption of PMs and PAMs for Cd
(II) and Pb (II) are shown in Figure 6. The results
indicated that the adsorption capacity of PAMs for
Pb (II) was higher than that of Cd (II) in the binary
metal ion solution. Moreover, the selectivity of
PAMs for Pb (II) is superior to PMs.
3.6 Desorption and Regeneration
The adsorption-desorption tests were repeated five
times in a binary ion mixture system. The results are
shown in Figure 7. After the 5th cycle, the adsorption
capacity of the PMs and PAMs for Pb (II) dropped to
15.66% and 9.64%, respectively, and remained
constant almost after the second cycle. However, the
adsorption capacities of PAMs for Pb (II) were even
143.47 mg·g
-1
with the existence of Cd (II). PAMs’s
adsorption capacities was about 2.2 times that of
PMs’s at the 5th recycle. These results indicated that
PAMs were of desirable reusability and stable
chemical property.
Figure 6: Effects of contact time on adsorption for Cd (II)
and Pb (II).
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
304
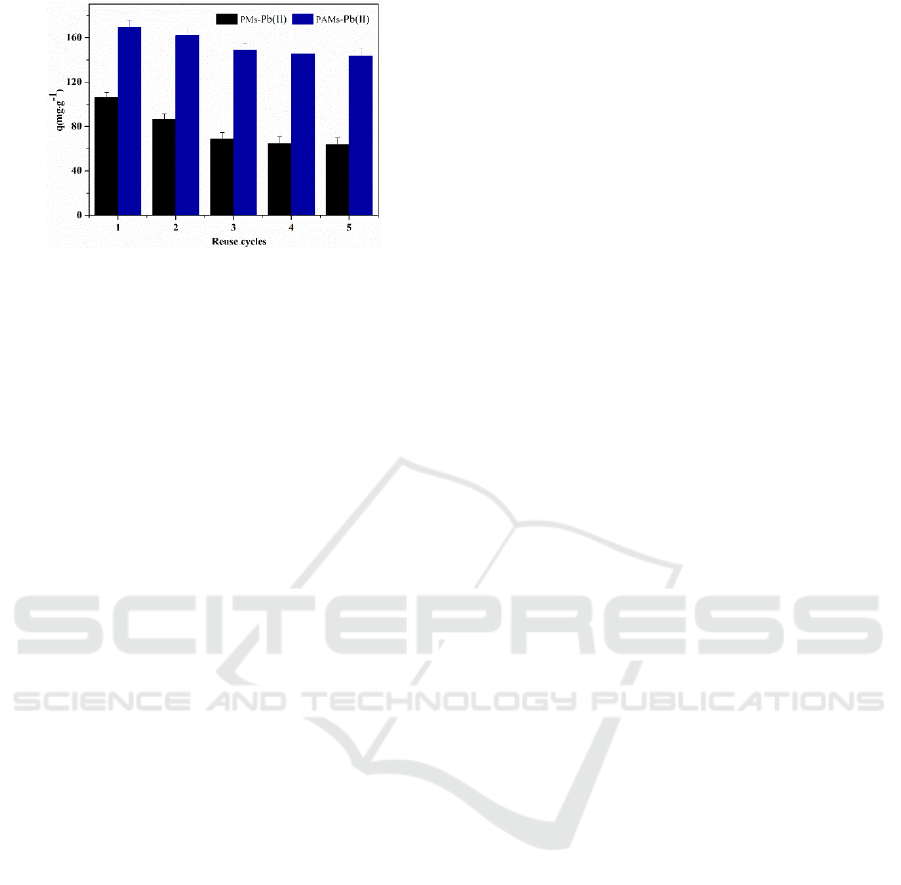
Figure 7: Results of five consecutive adsorption-desorption
for the reuse of microspheres for Pb (II) adsorption.
4 CONCLUSION
In this work, PMs and PAMs were successfully
fabricated through crosslinking with calcium ions.
The maximum adsorption capacity for Pb (II) of
PAMs was about 2.6 times of PMs which implied that
the adsorption ability of PAMs greatly increased.
And the selective adsorption capacity for Pb (II) of
PAMs was better than that of PMs. Moreover, the
result of regeneration experiments showed that the
removal efficiency for Pb (II) of PAMs was more than
54% after five adsorption-desorption cycles in a
binary ion mixture system. All the results above
implied that the newly-prepared PAMs might be the
promising adsorbent for Pb (II) in aqueous solutions.
ACKNOWLEDGEMENTS
This work was supported by Scientific Research
Foundation for Returned Scholars (Ministry of
Education of China, 201503), Opening Foundation of
Key Laboratory of Resource Biology and
Biotechnology in Western China (Northwest
University, Ministry of Education),Natural Science
Basic Research Plan in Shaanxi Province of China
(2017JM2016) and National Natural Science
Foundation of China (81673115).
REFERENCES
Afanas’Ev, S. P., Panova, É. P., Katseva, G. N., Kukhta, E.
P., and Chirva, V. Y., 1984. Modification of the
titrimetric method of analyzing pectin substances.
Chemistry of Natural Compounds, 20(4): 404-406.
Celus, M., Kyomugasho, C., Kermani, Z. J., Roggen, K.,
Loey, A. M. V., Grauwet, T., and Hendrickx, M. E.,
2017. Fe
2+
adsorption on citrus pectin is influenced by
the degree and pattern of methylesterification. Food
Hydrocolloids, 73: 101-109.
Chen, X., Zhang, W., Luo, X., Zhao, F., Li, Y., Li, R., and
Li, Z., 2017. Efficient removal and environmentally
benign detoxification of Cr(VI) in aqueous solutions by
Zr(IV) cross-linking chitosan magnetic microspheres.
Chemosphere, 185: 991-1000.
Chojnacka, K., 2010. Biosorption and bioaccumulation –
the prospects for practical applications. Environment
International, 36(3): 299-307.
España, J. S., Pamo, E. L., Pastor, E. S., Andrés, J. R., and
Rubí, J. A. M., 2006. The Removal of Dissolved Metals
by Hydroxysulphate Precipitates during Oxidation and
Neutralization of Acid Mine Waters, Iberian Pyrite Belt.
Aquatic Geochemistry, 12(3): 269-298.
Fonseca, M. G. D., Oliveira, M. M. D., Arakaki, L. N. H.,
Espinola, J. G. P., and Airoldi, C., 2005. Natural
vermiculite as an exchanger support for heavy cations
in aqueous solution. Journal of Colloid & Interface
Science, 285(1): 50-55.
Ilse, F., Eugénie, D., Thomas, D., Paula, M., Ann, V. L., and
Marc, H., 2009. Influence of intrinsic and extrinsic
factors on rheology of pectin-calcium gels. Food
Hydrocolloids, 23(8): 2069-2077.
Jorgetto, A. D. O., Silva, A. C. P. D., Wondracek, M. H. P.,
Silva, R. I. V., Velini, E. D., Saeki, M. J., Pedrosa, V. A.,
and Castro, G. R., 2015. Multilayer adsorption of Cu(II)
and Cd(II) over Brazilian Orchid Tree ( Pata-de-vaca )
and its adsorptive properties. Applied Surface Science,
345: 81-89.
Liu, L., Fishman, M. L., Kost, J., and Hicks, K. B., 2003.
Pectin-based systems for colon-specific drug delivery
via oral route. Biomaterials, 24(19): 3333-3343.
Martinez, Y. N., Piñuel, L., Castro, G. R., and Breccia, J.
D., 2012. Polyvinyl Alcohol–Pectin Cryogel Films for
Controlled Release of Enrofloxacin. Applied
Biochemistry & Biotechnology, 167(5): 1421-1429.
Mohammadi, T., Moheb, A., Sadrzadeh, M., and Razmi, A.,
2005. Modeling of metal ion removal from wastewater
by electrodialysis. Separation & Purification
Technology, 41(1): 73-82.
Mortaheb, H. R., Zolfaghari, A., Mokhtarani, B., Amini, M.
H., and Mandanipour, V., 2010. Study on removal of
cadmium by hybrid liquid membrane process. Journal
of Hazardous Materials, 177(1–3): 660-667.
Serguschenko, I., Kolenchenko, E., and Khotimchenko, M.,
2007. Low esterified pectin accelerates removal of lead
ions in rats. Nutrition Research, 27(10): 633-639.
Shariful, M. I., Sharif, S. B., Lee, J. J. L., Habiba, U., Ang,
B. C., and Amalina, M. A., 2017. Adsorption of
divalent heavy metal ion by mesoporous-high surface
area chitosan/poly (ethylene oxide) nanofibrous
membrane. Carbohydrate Polymers, 157: 57-64.
Adsorption of Pb (II) from Aqueous Solutions by Pectic Acid Microspheres
305
