
Keywords: Copper nanowires, hydrothermal
Abstract: In this paper, copper nanowires with different aspect ratios were synthesized by hydrothermal method at
150 ℃, using environmentally friendly and inexpensive reagents such as chloride dihydrate (CuCl
2
· 2H
2
O),
tetradecylamine (TDA), and glucose. Moreover, the effects of different reaction times and reactant ratios on
the synthesis were investigated to obtain copper nanowires with different aspect ratios spanning from 150 to
500.
1 INTRODUCTION
Copper nanowires are becoming increasingly
popular due to their advantages of good electrical
conductivity, low cost and abundant crustal content.
The applications of copper nanowires mainly depend
on their different aspect ratios. For example, copper
nanowires with low aspect ratio can be used for
catalytic reactions(He et al., 2014) and antimicrobial
applications(Jiang et al., 2015). High aspect ratio
copper nanowires can be used as transparent
electrodes(Guo et al., 2013) and they are also
applied in solar cells(Yu et al., 2016), organic light
emitting diodes(Eritt et al., 2010), and smart
windows(Runnerstrom et al., 2014). Therefore, it is
necessary to control the aspect ratio of the
synthesized copper nanowires. Zhang et al.
synthesized ultrathin semicircle-shaped copper
nanowires with the aspect ratio of around 2000,
which can be used in optical devices(Zhang et al.,
2018). Deshmukh et al. obtained high aspect ratio
copper nanowires which were used to fabricate
copper nanowire films with a sheet resistance of
24.5 Ω/sq, and a transmittance of T =
71%(Deshmukh et al., 2018). Wang et al.
successfully prepared copper nanowires by
hydrothermal method with aspect ratio of
approximately 2500(Wang et al., 2018). In this
paper, we prepared copper nanowires by a
hydrothermal method in an environmentally friendly
approach and investigated the effects of different
parameters in order to achieve controllable synthesis
of copper nanowires.
2 EXPERIMENTAL
2.1 Materials
Chloride dihydrate (Aladdin, AR), tetradecylamine
(Aladdin, 96%), and glucose (Aladdin, AR) were
used for the synthesis of copper nanowires.
Trichloromethane (Aladdin, 96%), ethanol (Aladdin,
99.7%), and hexane (Aladdin, 97%) were used as
solvents in centrifugal purification.
2.2 Synthesis of Copper Nanowires
Firstly, 0.34 g of copper chloride dihydrate, 0.36 g
of glucose and 1.6 g of tetradecylamine were
dissolved in 80 ml of deionized water. Then, the
mixture was stirred magnetically for half an hour.
Secondly, the solution was poured into a 100 ml
reaction tank, then charged into the reaction vessel,
and reacted at 150 ° C for 4 hours. Finally, after the
resulting solution was cooled down, the supernatant
was poured off, and the remaining red fibrous
material was collected. The fibrous material was
purified by centrifugation with deionized water, n-
hexane and chloroform, respectively. Finally, a pure
red material was obtained and stored in n-hexane.
Controllable Synthesis of Copper Nanowires by Hydrothermal
Method
Jinfeng Liu, Xiaohong Wang and Xiuqing Gong
*
Materials Genome Institute, Shanghai University, Chengzhong road, Shanghai 201800, China
Liu, J., Wang, X. and Gong, X.
Controllable Synthesis of Copper Nanowires by Hydrothermal Method.
DOI: 10.5220/0008187602030207
In The Second International Conference on Mater ials Chemistry and Environmental Protection (MEEP 2018), pages 203-207
ISBN: 978-989-758-360-5
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
203

Figure 1: SEM images of CuNWs. a. nCu : nglucose = 1:2; b. nCu : nglucose = 1:1; c. nCu : nglucose = 3:2; d. nCu :
nglucose = 2:1.
3 RESULTS AND DISCUSSION
3.1 Effect of Reactant Ratio
The effect of reactant ratios on the reaction was
investigated, especially the ratio between copper
chloride dihydrate and glucose. The amounts of
tetradecylamine and copper chloride dihydrate were
kept constant while the amount of glucose was
changed. The copper nanowires were obtained at the
same reaction time and reaction temperature.
Figure 1 exhibits the SEM images of CuNWs
synthesized with different reagent ratios. The
different reactant ratios caused the copper nanowires
to exhibit different morphologies. As seen from the
images in Figure 1, copper nanowires were formed
as the amount of glucose decreased, but several
copper crystals were also observed in Figure 1d. The
reason for this behaviour could be that : the
presence of five hydroxyl groups in one glucose
molecule, and the ratio of Cu2+ to glucose
molecules should be 1:0.4 in theory. The amount of
steric hindrance agents remained constant, so the
directional growth of copper crystals was limited. As
a result of decreasing ratio of glucose, less copper
nanoparticles were obtained and they were not fully
elongated along the [110] direction(Jin et al., 2011)
to generate CuNWs conforming to the Ostwald
ripening process. This also explained why addition
of less glucose resulted in the formation of thinner
and shorter CuNWs (as described below). In order to
study the difference in morphology, 50 to 100
copper nanowires with different reactant ratios were
measured, their length was tested, and finally their
aspect ratio was calculated, as shown in Figure 2.
Figure 2 illustrates the effect of different reactant
ratios on the diameter of copper nanowires. As the
amount of glucose decreased, there was an overall
trend of decreasing diameter, which was because
less CuNPs led to incomplete growth. Therefore, the
copper nanowires with different aspect ratios can be
obtained.
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
204
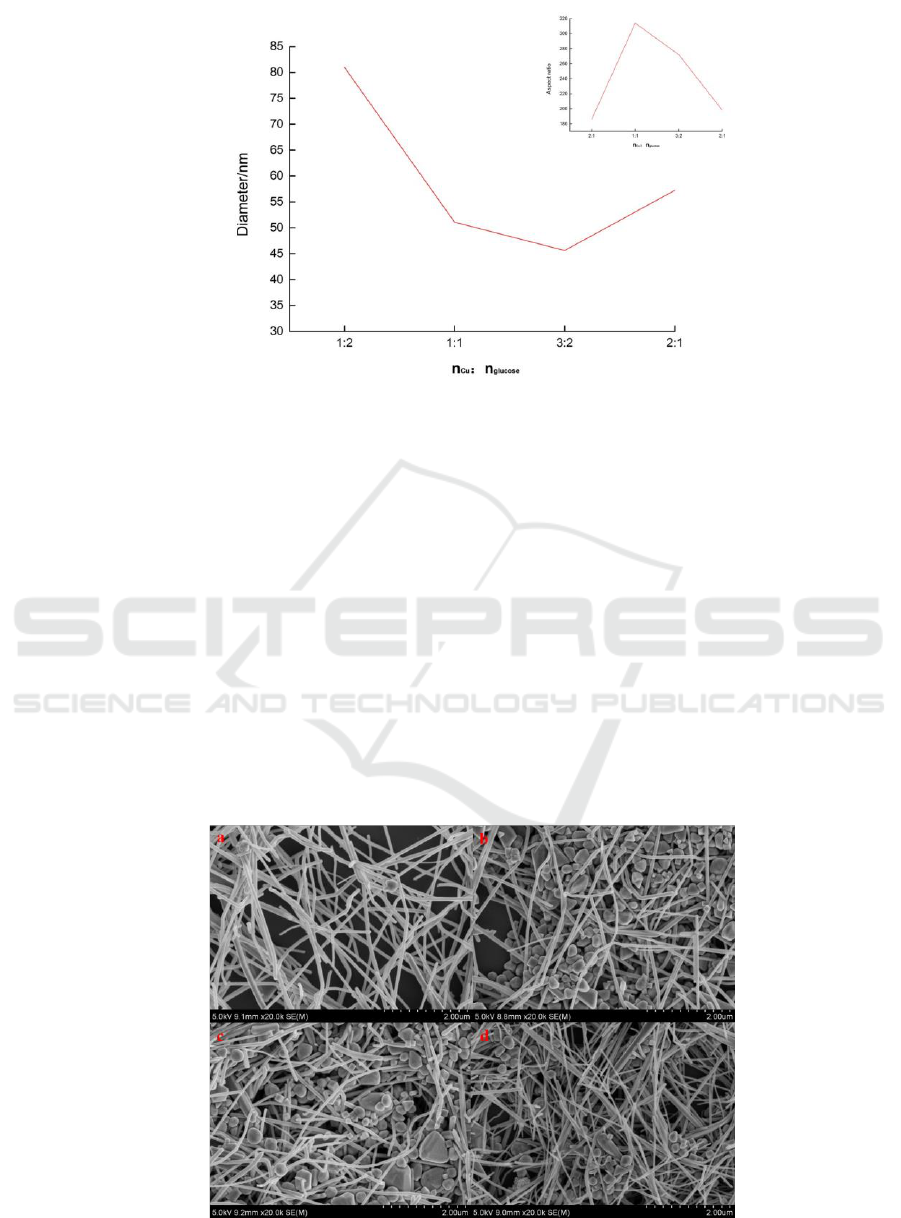
Figure 2: Change in diameter of copper nanowires with different reactant ratios. The inset is a line chart indicating the
relationship between the reactant ratio and the aspect ratio.
3.2 Effect of Reaction Time
The effect of reaction time on the synthesis was also
investigated, by keeping the ratio of reactants the
same, and changing the reaction time to 4h, 8h, 16h,
24h, and 32h.
Figure 3 shows the scanning electron
micrographs of copper nanowires prepared at
different reaction times. CuNPs grew in a particular
orientation due to the selective binding of steric
agent TDA to {100} facets of Cu (Jia et al., 2013).
The amount of TDA was constant, indicating limited
steric effects, and a prolonged reaction time led to a
more complete growth of CuNPs. In theory, the
purity of CuNWs should be better with increase in
reaction time. However, a small of amount of
CuNPs were observed in Figure 3b, Figure 3c and
Figure 3d, which could be due to an insufficient
purification process.
As seen in Figure 4, as the reaction time
increased, the diameter of the copper nanowires
decreased and the aspect ratio increased. When the
reaction time increased, the time of oriented growth
became longer. As a result, the length of copper
nanowires increased, and the diameter decreased as
the amount of reactants remained constant, which is
consistent with Ostwald ripening.
Figure 3: SEM images of CuNWs prepared with different reaction times. a. 8h; b. 16h; c. 24h; d. 32h.
Controllable Synthesis of Copper Nanowires by Hydrothermal Method
205
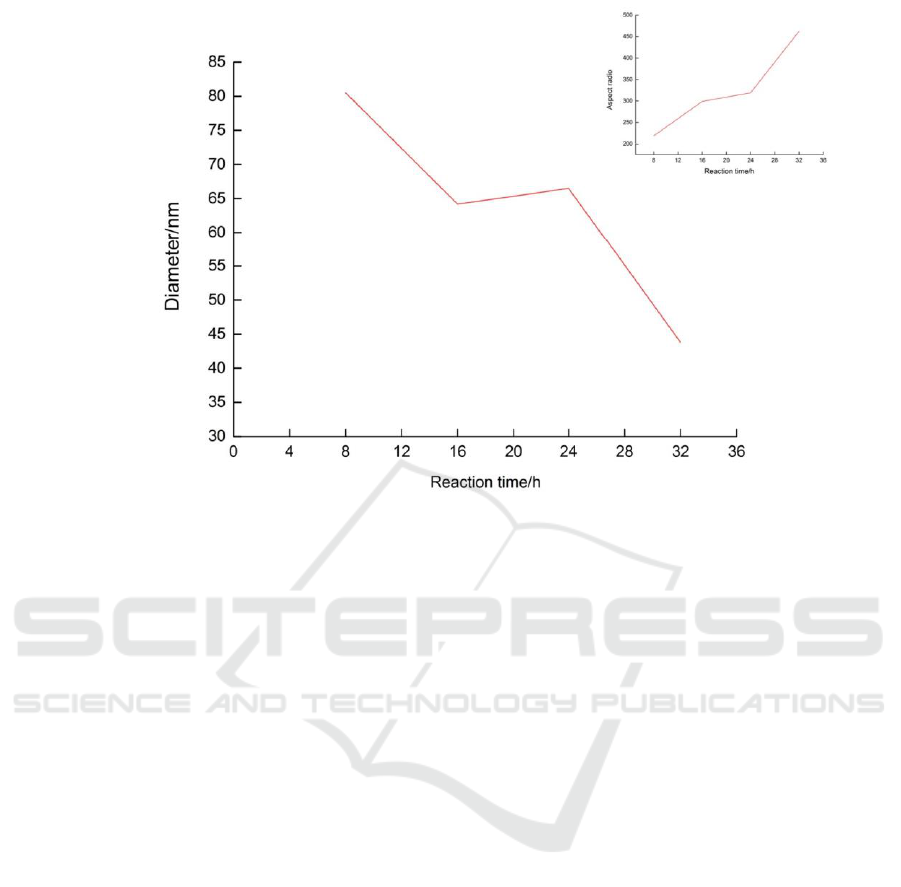
Figure 4: Change in diameter of copper nanowires with different reaction times. The inset is a line chart indicating the
relationship between the reaction time and the aspect ratio.
4 CONCLUSIONS
In this work, copper nanowires were controllably
prepared by a TDA-assisted hydrothermal method.
Moreover, the effects of different reactant ratios and
reaction times on the morphology of the resulting
copper nanowires were investigated. By varying the
above reaction parameters, CuNWs were obtained
with different aspect ratios ranging from 150 to 500.
When the amount of glucose decreased and reaction
time increased, thinner CuNWs were obtained. Thus,
the results of this work could help guide the
production of suitable CuNWs for different
applications such as sensors and solar cells.
ACKNOWLEDGEMENTS
This work was sponsored by the Shanghai Pujiang
Program (17PJ1402800) and the National Natural
Science Foundation of China (21775101).
REFERENCES
Deshmukh, R., Calvo, M., Schreck, M., Ternoort, E.,
Sologubenko, A. S. & Niederberger, M. 2018.
Synthesis, Spray Deposition, and Hot-Press Transfer
of Copper Nanowires for Flexible Transparent
Electrodes. ACS Appl Mater Interfaces, 10, 20748-
20754.
Eritt, M., May, C., Leo, K., Toerker, M. & Radehaus, C.
2010. OLED manufacturing for large area lighting
applications. Thin Solid Films, 518, 3042-3045.
Guo, H., Lin, N., Chen, Y., Wang, Z., Xie, Q., Zheng, T.,
Gao, N., Li, S., Kang, J., Cai, D. & Peng, D.-L. 2013.
Copper Nanowires as Fully Transparent Conductive
Electrodes. Scientific Reports, 3, 2323.
He, R., Wang, Y.-C., Wang, X., Wang, Z., Liu, G., Zhou,
W., Wen, L., Li, Q., Wang, X., Chen, X., Zeng, J. &
Hou, J. G. 2014. Facile synthesis of pentacle gold–
copper alloy nanocrystals and their plasmonic and
catalytic properties. Nature Communications, 5, 4327.
Jia, B., Qin, M., Zhang, Z., Chu, A., Zhang, L., Liu, Y. &
Qu, X. 2013. The influence of reagents on the
preparation of Cu nanowires by tetradecylamine-
assisted hydrothermal method. Journal of Materials
Science, 48, 4073-4080.
Jiang, S., Sreethawong, T., Lee, S. S. C., Low, M. B. J.,
Win, K. Y., Brzozowska, A. M., Teo, S. L.-M.,
Vancso, G. J., JAŃCZEWSKI, D. & Han, M.-Y. 2015.
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
206

Fabrication of Copper Nanowire Films and their
Incorporation into Polymer Matrices for Antibacterial
and Marine Antifouling Applications. Advanced
Materials Interfaces, 2, 1400483.
Jin, M., He, G., Zhang, H., Zeng, J., Xie, Z. & Xia, Y.
2011. Shape-Controlled Synthesis of Copper
Nanocrystals in an Aqueous Solution with Glucose as
a Reducing Agent and Hexadecylamine as a Capping
Agent. Angewandte Chemie International Edition, 50,
10560-10564.
Runnerstrom, E. L., Llordes, A., Lounis, S. D. & Milliron,
D. J. 2014. Nanostructured electrochromic smart
windows: traditional materials and NIR-selective
plasmonic nanocrystals. Chem Commun (Camb), 50,
10555-72.
Wang, Y., Liu, P., Zeng, B., Liu, L. & Yang, J. 2018.
Facile Synthesis of Ultralong and Thin Copper
Nanowires and Its Application to High-Performance
Flexible Transparent Conductive Electrodes.
Nanoscale Res Lett, 13, 78.
Yu, P., Wu, J., Liu, S., Xiong, J., Jagadish, C. & Wang, Z.
M. 2016. Design and fabrication of silicon nanowires
towards efficient solar cells. Nano Today, 11, 704-737.
Zhang, Y., Guo, J., Xu, D., Sun, Y. & Yan, F. 2018.
Synthesis of ultrathin semicircle-shaped copper
nanowires in ethanol solution for low haze flexible
transparent conductors. Nano Research, 11, 3899-3910.
Controllable Synthesis of Copper Nanowires by Hydrothermal Method
207
