
The Spectra Study on Degradation of Sarin Simulant Diisopropyl
Fluorophosphate by Plasma Coupled with Ozone
Hongjie Zhao, Zhen Hu and Zhanguo Li
*
State Key Laboratory of NBC protection for Civilian, Beijing 102205, China
Keyword: dielectric barrier discharge(DBD), plasma, O
3
, diisopropyl fluorophosphate, reactive particles, diagnosis of
spectrometry
Abstract: The diagnosis of irradiance particles produced in the plasma degradation of diisopropyl fluorophosphates
(DFP) was studied by spectral method. The characteristic peaks of [O],[O
+
],[O
2
+
] were obtained by
analyzing spectra intensity changes in condition of different discharge parameters. As a result, it is found
that the intensity of [O] peaks disappear when DFP is involved in the reaction. Moreover, for [O
+
] peaks,
the intensity drop of 398.69 nm was 70.1%, and other three peaks disappeared, and for [O
2
+
] peaks, the
intensity drops of 226.49 nm and 231.19 nm were 95.3% and 94.5%, and 216.92 nm peak disappeared,
which means [O], [O
+
] and [O
2
+
] play important roles in the degradation of DFP by plasma.
1 INTRODUCTION
It is well known that dielectric barrier discharge
plasma is mainly high-energy electrons and free
radicals for the degradation of pollutants (Yu et al.,
2015). In order to diagnose the active particles in
plasma system, a characteristic peak set of emission
spectra is established for several common active
particles to determine the type and concentration of
the produced active particles, and the concentration
change of the active particles is used to assist in
confirming the mechanism of plasma degradation of
pollutants. Currently, probe, wave
interference(Chang et al., 2007), mass
spectrum(Jasmine et al., 2018) and spectrum
technologies have been used to spectra diagnosis of
plasma. Among these technologies, spectra
diagnosis is a common and simple measuring
technology(Bibhuti et al., 2017; Saeed et al., 2014).
Much spectra researches of plasma have been
reported and more about the pure gas, such as the
pure oxygen(Zlatko et al., 2011) and nitrogen(Yan,
2004). But the spectral research of mixed gases
(Andriy et al., 2016) is more difficult, because there
are too many spectral lines to distinguish. There is
few spectral analysis to the process of disinfection
reaction, so a set of principles for spectral analysis
has been formulated and applied to plasma reaction
to confirm the active particles involved in the
reaction. So, spectral analysis is used to assist the
analysis of plasma reaction.
2 EXPERIMENT
2.1 Experimental Setup
Reactor:The outer electrode of non-thermal DBD
plasma reactor is ground electrode (outer diameter
45 mm, wall thickness 15 mm), and coaxial inner
aluminium electrode is used as the high voltage
electrode (diagram 10 mm). The medium media is a
quartz glass tube (outer diagram 15 mm, wall
thickness 1 mm) is used as dielectric and fixed
between inner and outer electrode, and the gap
between high voltage electrode and quartz glass tube
is 1.5 mm. The discharged gas enters the reactor
through the inner electrode, and then exhausts from
the gap between inner electrode and quartz glass
tube. The reactor is put in the spectrum measuring
cavity to avoid the interference of outside. The fiber
probe of spectrometer is put 1cm from the electrode
in the quartz glass tube of reactor.
Power: High frequency power is employed that
based on the atmospheric DBD plasma, and the
power parameters is adjustable continuously. The
range of pulse voltage peak value: 0-20 KV; the
range of pulse current peak value: 0-200 mA; the
Zhao, H., Hu, Z. and Li, Z.
The Spectra Study on Degradation of Sarin Simulant Diisopropyl Fluorophosphate by Plasma Coupled with Ozone.
DOI: 10.5220/0008185500590062
In The Second International Conference on Materials Chemistry and Environmental Protection (MEEP 2018), pages 59-62
ISBN: 978-989-758-360-5
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
59

range of pulse frequency: 0-15 MHz; the range of
average export power: 0-500 W. These parameters
are detected by oscillograph (TDS1012B-type, fitted
P6015A-1000X-type high voltage detector and
A621-type current detector, America Tektronix
Company) in real time.
2.2 Analysis Method
The quantitative analysis of DFP is operated by GC
(7890A-type, fitted FPD detector, Agilent
Company). The analysis of active particles is
operated by spectrometer (AvanSpec-2048 type,
fitted fiber probe, Avantes Company).
The plasma reactor and the spectral probe are
placed in the spectral detection chamber, and the
fiber probe is fixed in the plasma reactor quartz
medium tube. The analysis process is as follows.
Firstiy, open the AvanSpec-2048 spectrometer and
perform parameter setting (integration time, etc.).
Secondly, the pure gas spectrum is detected.
Thirdly, the air discharge spectrum is detected, thus
the type and amount of the active particles are
determined. Finally, spectral detection was
performed before and after the air plasma
degradation reaction.
3 RESULTS AND DISCUSSION
3.1 Analysis of Spectra
To study the spectral changes of active particles
during plasma degradation of DFP, this study
intends to determine the characteristic peaks of
several common active particles such as
[O],[O
+
],[O
2
+
] by the emission spectrum
experiments and literature review of pure gas (N
2
,
O
2
, Ar) and air environment. Due to the plasma
reaction, particle level structure and interference
between particles, the paper believes that the
selection of characteristic peaks of active particles
should meet the following principles:
Firstly, the peaks are stably present in the plasma
of different background gases, which is the basis for
determining the characteristic peaks of the active
particles; Secondly, the intensity of the peak is
sensitive to the discharge power. The amount of
active particles is closely related to the input energy.
The active particles may have dozens of lines. Some
of the lines may vary with energy, and the intensity
changes are not obvious. Therefore, it is necessary
to select those spectral lines that are more sensitive
to energy (power) as characteristic peaks; Thirdly,
the spectral intensity is high and easy to distinguish;
Fourthly, the spectrum peak is away from
characteristic peaks of other active particles.
Overlapping peaks may exist because of many
spectrum lines from different active particles. If the
location of two peaks is nearer, they may overlap
affected by resolution ratio.
Take [O] for example, according to spectrum
data comparison between literatures (Xie, 2008;
Zhang et al., 2002) and this research, approximative
wavelength peaks were searched. The principles
were also used to treat these peaks.
(1) The spectrum character of [O] in discharging
condition of pure oxygen
The spectrum of pure oxygen (gas flow 100 L/h)
with the DBD plasma was detected in three different
power of 74.4 W, 119.2 W and 154.8 W. comparing
with literature, 15 rather apparent peaks of [O]
(394.75 nm,395.38 nm,543.50 nm,700.19 nm,700.77
nm,700.82 nm,725.17 nm,725.39 nm,747.64
nm,777.28 nm,778.11 nm,795.63 nm,822.53
nm,844.73 nm,845.08 nm) were discovered. 5
spectrum peaks (543.50 nm,700.77 nm,700.82
nm,822.53 nm,844.73 nm) that its intensity is
stronger with the power rising were selected by
comparing the spectrum intensity of different power,
as is shown in Table 1.
Table 1: The spectrum intensity of [O] in discharging
condition of pure oxygen with different power.
Wavelength
/nm
Level
74.4
W
119.2
W
154.8
W
1.0:1.6:2.1
The spectrum
intensity ratio under
condition of three
power
543.50
5
S
0
-
5
P
78.4
101.6
135.8
1.0
1.3
1.7
700.77
3
P -
3
D
0
173.6
174.4
190.4
1.0
1.0
1.1
700.82
3
P -
3
D
0
104.2
113.8
133.0
1.0
1.1
1.3
822.53
3
D
0
-
3
D
73.0
77.8
103.4
1.0
1.1
1.4
844.73
3
S
0
-
3
P
47.4
65.6
140.6
1.0
1.4
3.0
Note: Regarding the spectra intensity value of the lowest power
as basis, and divided by all spectra intensity value of different power
of each wavelength. This is the calculation method of spectra intensity
ratio. Take 543.5 nm, for example, 78.4/78.4=1.0,101.6/78.4=1.3,
135.8/78.4=1.7. The style of level is low level-high level.
The 5 peaks are sensitive to the power and
stronger with the power rising. The energy gap of
excited state and ground state is different. After
eradiated transition, peaks of different wavelength
are emitted. Spectrum intensity of 700.77 nm is
insensitive to power, while the intensity of other
four peaks is stronger with the power rising. The
energy requirement of excited state is different with
different energy levels. Jump to the lower level
5
S
0
,
3
S
0
, the intensity ratio of 543.50 nm and 844.73
nm peaks is 1.0:1.3:1.7 and 1.0:1.4:3.0 when the
power ratio is 1.0:1.6:2.1. So the intensity change is
obvious. The reason for the difference of spectrum
intensity ratio of different peaks is the quantum
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
60
yield of eradiated transition between different
excited state and ground state different, which leads
to the intensity change of spectrums.
(2) The spectrum character of [O] in discharging
condition of Ar.
In order to confirm the stable existence of
characteristic peaks of [O] in different conditions,
the discharging experiment of pure Ar+1.8%O
2
(gas
flow 100 L/h) with the DBD plasma was made in
condition of three different power 41.2W, 65.5W,
120.0W.
(3) The spectrum character in discharging
condition of air
The spectrum of air (gas flow 100L/h) with the
DBD plasma was detected in condition of three
different power 107W, 136W, 198W. The spectrum
intensity ratio of 5 peaks (detected from pure
oxygen spectrum) in discharging condition of air
was calculated.
In conclusion, 543.50nm, 700.77nm, 844.7nm
were selected as the characteristic spectrum peaks of
[O]. According to the principles of characteristic
peaks, the characteristic peaks of [O
+
], [O
2
+
]were
398.69nm, 410.49nm,467.68nm, 494.10nm and
216.92nm, 226.49nm, 231.19nm.
3.2 Analysis of the Roles for Active
Particles in the Degradation of DFP
O
3
is a strong oxidant. But the active particles of O
3
produced in plasma play a more important role,
rather than the strong oxidability of itself. The
molecule iron of O
3
was not reported and this paper
also did not find the molecule iron in O
3
spectrum
experiment. So we infer that the active particles of
O
3
is similar to O
2
.
To confirm the active particles that participate in
DFP degradation, the spectrum of air +O
3
discharging plasma and air+O
3
+DFP discharging
plasma was detected. The spectrum before and after
degradation was compared and analyzed in
condition of O
3
concentration 500 mg/m
3
, initial
concentration of DFP 19.5 mg/m
3
, flow 400 L/h,
power 105 W.
(1) Effects of [O]
[O] can be produced by the following process:
e + O
3
→ O
2
+ O + e e + O
2
→ 2O +e (1)
e + O
2
→ O +O
-
e +O
2
→ O +O
+
+2e (2)
According to the comparison of spectrum before
and after DFP degradation, it is found that the
spectrum peaks of [O] existed in the spectrum of
plasma without DFP degradation (the magnifying
part of Figure 1) obviously, while the peaks was not
detected in the spectrum of plasma with DFP
degradation reaction(the magnifying part of Figure
2). Which means [O] did not exist or its
concentration was under the limit of detection
(LOD) of spectrometer. The only changed factor of
experiment was the addition of DFP, and its
concentration reduced, so the strong oxidizing [O]
was consumed in process of DFP degradation.
(2) Effects of [O+]
[O+] can be produced by the following process:
e + O
3
→O
2
+ O + e e + O
2
→ O + O
+
+ 2e (3)
e + O
2
→ O
+
+ O
-
+ e e + O → O
+
+ 2e (4)
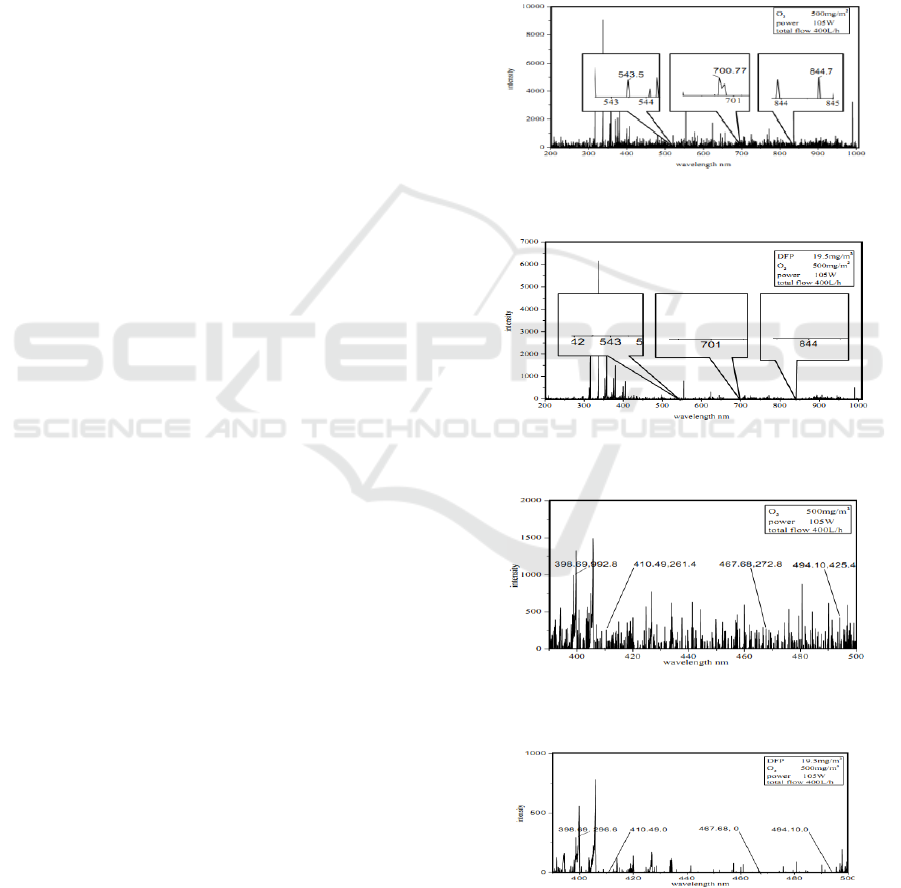
Figure 1: The spectrum of [O] by plasma coupled with O
3
.
Figure 2: The spectrum of [O] from DFP degradation by
plasma coupled with O
3
.
Figure 3: The spectrum of [O
+
] from plasma coupled with
O
3
.
Figure 4: The spectrum of [O
+
] from DFP degradation by
plasma coupled with O
3
.
The Spectra Study on Degradation of Sarin Simulant Diisopropyl Fluorophosphate by Plasma Coupled with Ozone
61
The spectrum before and after the DFP
degradation was shown in Figure 3 and Figure 4.
According to the contrast of 4 spectrum peaks of
[O
+
], the intensity drop of 398.69 nm was 70.1%,
and other three peaks disappeared. The
concentration of [O
+
] reduced apparently. So [O
+
]
was one of main particles in DFP degradation.
(3) Effects of [O
2
+
]
[O
2
+
] can be produced by the following
process:
e + O
2
→ O
2
+
+ 2e O
+
+ O
2
→ O
2
+
+ O (5)
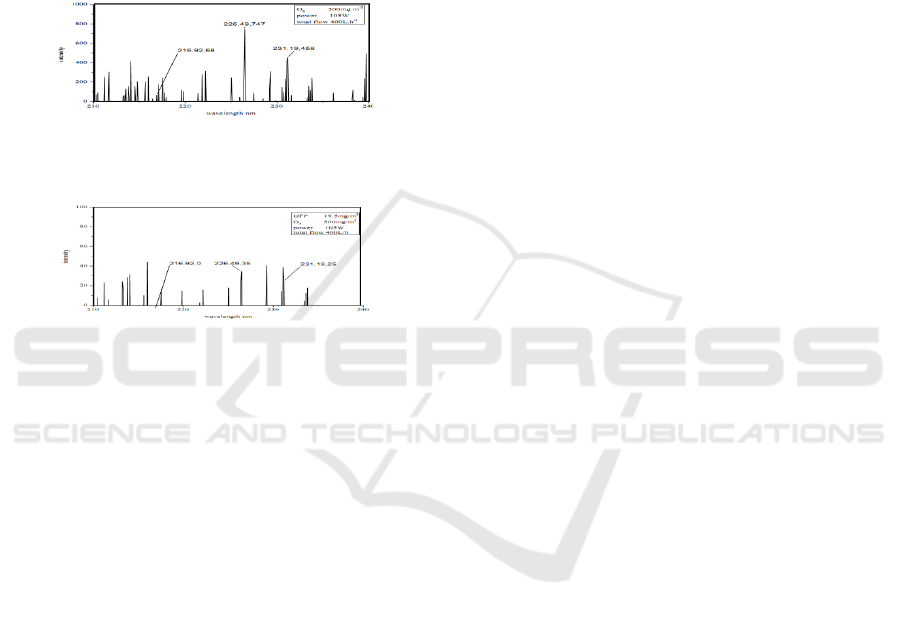
Figure 5: The spectrum of [O
2
+
] from plasma coupled with
O
3
.
Figure 6: The spectrum of [O
2
+
] from DFP degradation by
plasma coupled with O
3
.
The spectrum before and after the DFP
degradation was shown in Figure 5 and Figure 6.
According to the contrast of 3 spectrum peaks of
[O
2
+
], the intensity drops of 226.49 nm and 231.19
nm were 95.3% and 94.5%, and 216.92 nm peak
disappeared. That means the concentration of [O
2
+
]
reduced apparently. So [O
2
+
] was one of main
particles in DFP degradation.
In conclusion, [O],[O
+
] and [O
2
+
] played an
important role in DFP degradation process with
plasma coupling O
3
.
4 CONCLUSIONS
This paper put forward a basic principle for
selecting characteristic peaks based on theory study
and experimental data analysis. The diagnosis of
irradiance particles produced in the plasma
degradation of DFP was studied by spectral method.
The characteristic peaks of [O], [O
+
], [O
2
+
] were
obtained by analyzing spectra intensity changes in
condition of different discharge parameters. As a
result, it is found that the intensity of [O] peaks
disappear when DFP is involved in the reaction.
Moreover, for [O
+
] peaks, the intensity drop of
398.69 nm was 70.1%, and other three peaks
disappeared, and for [O
2
+
] peaks, the intensity drops
of 226.49 nm and 231.19 nm were 95.3% and 94.5%,
and 216.92 nm peak disappeared, which means [O],
[O
+
] and [O
2
+
] play important roles in the
degradation of DFP by plasma.
REFERENCE
Andriy, K., Carsten, E., Klaus, K., et al., 2016.
Spectroscopic characterization of a low-temperature
plasma ambient ionization probe operated with
helium/nitrogen plasma gas mixtures. J. Anal. At.
Spectrom., 31, 1574-1581. DOI:10.1039/C6JA00148C
Bibhuti, B. S., Su, B. J. , Jeon, G. H., 2017. Development
and characterization of a multi-electrode cold
atmospheric pressure DBD plasma jet aiming plasma
application. J. Anal. At. Spectrom., 32, 782-795.
DOI:10.1039/C6JA00419A
Chang, C. H., Hsieh, C. H., Wang, H. T. et al., 2007. A
transmission-line microwave interferometer for
plasma electron density measurement. Plasma Sources
Sci Technol, 16:67. DOI:
10.1088/0963-0252/16/1/009.
Jasmine, S. F., Peter, C., Hauser, 2018. A low-cost
ambient desorption/ionization source for
mass-spectrometry based on a dielectric barrier
discharge. Analytical Methods, 10, 2701-2711.
DOI:10.1039/C8AY00446C
Saeed, A., Khan, A. W., Jan, F. et al., 2014. Optimization
Study of Pulsed DC Nitrogen-Hydrogen Plasma in the
Presence of an Active Screen Cage. Plasma Sci.
Technol., 16 (5): 460-464. DOI:
10.1088/1009-0630/16/5/04.
Xie, W. J., 2008. Spectroscopic Diagnostics of Active
Species and Their Environmental Chemical Process of
O2, N2, CO2, in Cold Plasma[Ph.D]. Shanghai
Jiaotong University, Shanghai.
Yan, Y., 2004. The experimental study of
N2(C3Πu→B3Πg) spectrum of pulse corona
plasma[Ph.D]. Dalian University of Technology,
dalian.
Yu, Y. L., Zhuang, Y. T., Wang, J. H., 2015. Advances in
dielectric barrier discharge-optical emission
spectrometry for the analysis of trace species.
Analytical Methods, 7, 1660-1666.
DOI:10.1039/C5AY00003C.
Zhang, Z. T., Bai, M. D., Zhou, X. J. et al. 2002. The
studies of the plasma process of ozone generation with
a strong ionization discharge and its application.
Technology and Equipment for Environmental
Pollution Control, 3(4): 26-31.
Zlatko, K., Marijan, B., Slobodan, M. et al., 2011.
Monitoring Oxygen Plasma Treatment of
Polypropylene With Optical Emission Spectroscopy.
IEEE TRANSACTIONS ON PLASMA SCIENCE,
39(5): 1239-1246. DOI: 10.1109/TPS.2011.2123111.
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
62
