
A Rapid Determination of Free Formaldehyde Content in Marine
Products
Xuan Zhang, Guangxin Yang, Cong Kong, Xiaosheng Shen, Youqiong Cai and Dongmei Huang
*
East China Sea Fishery Research Institute, Chinese Academy of Fishery Sciences, Key Laboratory of Control of Quality
and Safety for Aquatic Products, Ministry of Agriculture, Shanghai 200090
Keywords: formaldehyde; squid; liquid chromatography; 2,4-dinitrophenylhydrazine
Abstract: Objective for rapid detecting free formaldehyde (FA) in marine products, a method with high sensitivity and
little interference is described. Background value of FA in marine products would supplement the scientific
evaluation index system. Free FA is derivatised with 2,4-dinitrophenylhydrazine (DNPH) to form a
chromophore for high-performance liquid chromatography (HPLC) detection. The formation of the DNPH
FA derivative is shortened to 30 min. It shows good linear correlation between the peak areas and FA
concentrations with a dynamic linear range of 0.3-25.0 mg/L, the limit of detection (LOD) is 0.2 mg/Land the
limit of quantitation (LOQ) is 0.5 mg/L. The recovery range of free FA in spiked squid was 70%-78% with
relative standard deviation (RSD) of 5%-10% (n=5). FA content is detected in 14 species of seafood
comparing to the past analysis method, results show no significant difference, FA content of tuna, tuna, cod,
Surf Calm and cuttlefish is more than 40 mg/kg. The average of FA content in 28 species of packaging squid
products is 14.7 mg/kg, ranging from 2.10-61.8 mg/kg. This method is simpler and easier to operate; it reduces
the concentration of derivatives, shortens the reaction time, and is applicable to the determination of
formaldehyde content in all kinds of seafood.
1 INTRODUCTION
FA is a highly active gas with low molecular weight
at room temperature. It is sold in the form of formalin
(containing 6-13 percent of FA), used as preservative,
insecticide and acaricide in aquatic products. As a
toxic substance (Liteplo et al., 2003; Zhang et al.,
2018), FA is easy to react with nucleophilic material,
causing DNA damage (IARC, 2004). Thrasher &
Kilburn believes that FA could lead to fetal toxicity
and aberration (Thrasher and Kilburn, 2001). FA
ranked second on the priority control list of toxic
chemicals in China (Tang et al., 2009). In 2004, FA
was categorized in Group I as ‘carcinogenic to
humans’ by the International Agency for Research on
Cancer (IARC) (Noda et al., 2011) , The United
States Environmental Protection Agency
recommended daily intake of FA as no more than
0.2mg/kg of the body weight while WHO set it as
0.15mg/kg of the body weight. The American Cancer
Society considers that FA in the air, food and water is
a carcinogen. However, authorities of European Food
Safety believe that oral FA is not carcinogenicand the
oral reference dose is 0.2mg /kg (EFSA, 2006). In
1985, Italian health departments set limit of FA in cod
and shellfish aquatic products respectively 60 mg/kg
and 10 mg/kg (MINSAN-telegram, 1985). Chinese
Ministry of Agriculture set it to “no detectable” in
aquatic products in 2001, and 10 mg/kg in 2002, at
present, two standards have been abolished, and no
uniform standard of FA is put forward.
Detection methods of FA are spectrophotometry
(Yasri et al., 2015; Chen et al., 2018), HPLC (Lv et
al., 2010; Zhang et al., 2018) and gas chromatography
(GC) (Ma et al., 2015; Shao et al., 2015). In this study,
HPLC method was used because of its convenient
operation, high accuracy and high sensitivity. The
concentration, time and temperature of the derivative
reagent were optimized, and the chromatographic
conditions were optimized. At the same time, FA
content in seafood was determined by the improved
method.
Zhang, X., Yang, G., Kong, C., Shen, X., Cai, Y. and Huang, D.
A Rapid Determination of Free Formaldehyde Content in Marine Products.
DOI: 10.5220/0008185200470050
In The Second International Conference on Materials Chemistry and Environmental Protection (MEEP 2018), pages 47-50
ISBN: 978-989-758-360-5
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
47

2 EXPERIMENTAL SECTION
2.1 Chemicals and Equipment
Marine products: fresh squid was bought directly
from the returning ship, other seafoods were bought
from aquatic product market in Shanghai, and
seafoods were stored at -20 º C back to the lab. Squid
products were purchased from supermarkets.
Agilent Technologies 1100 HPLC (the USA)
consisted of a pump, a VU detect, a column chamber,
and an Agilent ChemStation for LC system; Millipore
water purification system (Millipore, the USA).
Acetonitrile was chromatographic pure (Baker,
the USA), DNPH and the rest of the reagents were
analytically pure; FA standard: 10 mg/mL, 2 mL
(Aladdin, Shanghai).
In addition, FA in aqueous solution could form a
stable hydrate with the formula H2C(OH)2: the
hydrate exists in equilibrium with various oligomers.
FA further forms an insoluble white trimer and
further polymerises to solid paraformaldehyde in
aqueous solutions. Sometimes even unopened bottles
of formalin had insoluble white precipitate. Therefor
we chose clear FA solution as the standard solution.
2.2 Experimentation
2.2.1 Preparation of Standard Solution and
Derivative Solution
FA standard solution (200 µg/mL): dissolved 2 mL of
FA standard and constant volume to 100 mL with
water, and the standard intermediate liquid could be
used for six months saved at 4 ºC.
The derivative solution: took 500 mg weight of
DNPH into 1 L acetonitrile, we got derivative liquid;
then took 5.28 g weight of sodium acetate into 2 mL
glacial acetic acid, and constant volume to 1 L with
water, we got buffer solution; 10 mL of each solution
was mixed to get the derivative solution.
2.2.2 Sample Derivatization and Extraction
For determining FA content, mixed (2+0.02) g
homogenized sample and 20.0 mL derivative liquid
in 50 mL polypropylene centrifuge tube, tighten the
plug, and then blent through vortex device, then put
in Water-bathing Constant Temperature Vibrator at
60 ºC, 150r/min for 30min. The mixture was filtered
through a 0.45um HV filter before injection. For each
sample five replicates were analyzed. Results were
expressed as mg of FA /kg.
2.2.3 Chromatographic Condition
The HPLC column was a Hypersil ODS-C18, 4.6 mm
×250 mm, 5 μm. The sample vol ume was set at 20
µl, the absorb wavelength of detector was set at 365
nm, the column temperature was set at 40 ºC. The
mobile phase was methanol-water (70:30, v/v) with a
flow rate of 0.9 mL/min. The peak area was used for
quantitative calculation of formaldehyde.
2.2.4 Calibration Curve
Respectively took 0.015, 0.025, 0.05, 0.25, 0.5, 1.0
mL FA standard solution (200 µg/mL) into 10 mL
volumetric flask, added buffer solution to 5 mL, and
derivative liquid to 10.0 mL, hence FA standard
solution was respectively diluted into 0.3, 0.5, 1.0,
5.0, 10.0, 50.0 µg/mL as FA work solution. The FA
work solution was derivatised and extracted
according to described procedures. Three injections
of each standard solution were made and the peak
area was the corresponding FA content to obtain the
calibration curve.
2.2.5 Data Processing
The data were statistically analyzed by Microsoft
Excel and the anova was analyzed by SPSS.
3 RESULTS AND DISCUSSION
3.1 Liquid Chromatographic Analysis
The Figure 1 showed that calibration curve in the 0.3-
25 µg/mL range was obtained and correlation
coefficient was 1. Figure 2 showed chromatogram of
5mg/kg of FA in squid sample by HPLC. The peak in
3.597min was residual DNPH, the other peak in
5.413min was considered to be a derivative of
HCHO-DNPH in squid. Table 1 showed that the
average recoveries of this method were in the range
of 70-78%, RSD was 5.3-10%.
Table 1: Recovery and precision data of FA (n=5).
Added[mg/kg]
Recovery[%]
RSD[%]
5
70
10
20
75
8.1
100
78
5.3
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
48
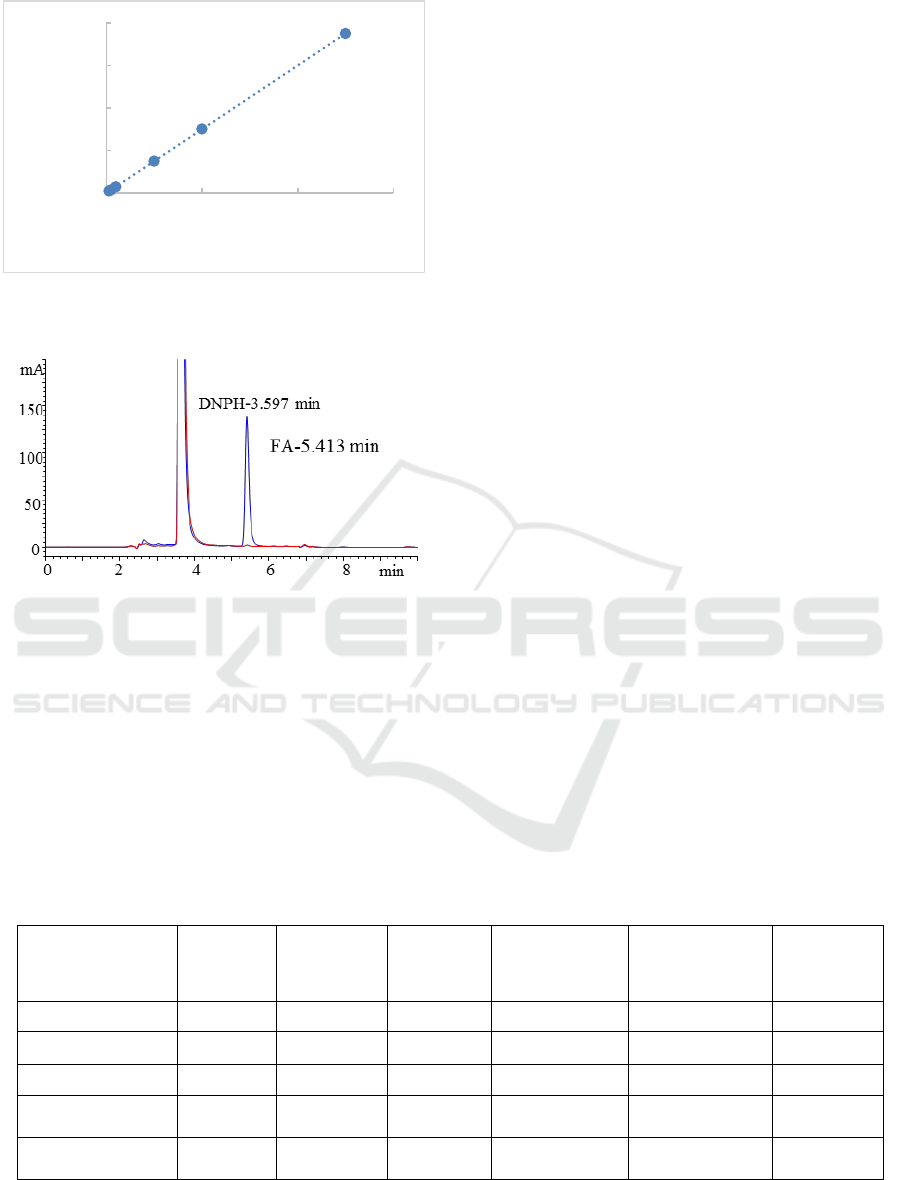
Figure 1: The Calibration Curve.
Figure 2: Chromatograms of the determination.
3.2 Discussion
3.2.1 The Concentration and States of FA in
Marine Products
Trimethylamine oxide (TMAO) widely exists in food,
TMAO could resolve into DMA, TMA and FA under
enzymolysis condition of trimethylaminek-N-oxide
(TMAOase). Besides the enzymatic pathway, FA is
steadily accumulated during the thermal processing
(Chen et al., 2017; Huang et al., 2017).
FA could react with protein, amino acid and
creatinine, which makes free and bound forms of FA
in organisms. “Total” formaldehyde is the sum of
these two forms. Bound FA could be extracted
through steam distillation under the sulfuric acid or
phosphoric acid solution (1% -40%). Therefore, it is
essential to specify whether free or bound
formaldehyde is being determined when reporting FA
content in tissue. Yeh et al. studied 10 different kinds
of marine products, they found that total content of
FA was 20mg/kg more than free FA, the proportion
of free content of FA ranged 39 percent among total
FA (Yeh et al., 2013). Rehbein et al. found that free
FA was 22.8 mg/kg ranged 19.9% among total FA in
cod, free FA was 7.6 mg/kg ranged 19.7% among
total FA in Haddock, free FA was 6.5 mg/kg ranged
15.5% among total FA in Pollack (Rehbein and
Schmidt, 1996). Literatures state that free FA is that
which is of toxicological interest and that it should be
measured (Bechmann, 1998). Low recovery is the
disadvantage of detecting free FA, so the authors used
a “recovery factor” (Treezl et al., 1997).
3.2.2 Detection Methods of Free FA
Detection methods of free FA include
spectrophotometry, chromatography, fluorescence
method, colorimetry and electrochemical method.
Generally spectrophotometry and chromatography
are used more, Table 2 Showed the comparation of
different methods of detecting free FA using DNPH.
The method in this paper was to react at room
temperature with simple operation, the results showed
high accuracy and sensitivity.
Table 2: Comparation of different methods.
References
Linearity
range
[mg/L]
LOD
[mg/L]
LOQ
[mg/L]
Derivative time
Derivative
temperature
Recovery
[%]
Zhang et al., 2018
0.5-50
0.3
0.5
60 min
60℃
63-74
Bechmann, 1998
-
0.00892
0.0268
Distillation
100 ℃
83-103
Treezl et al., 1997
0.05-2
0.005
0.05
15 min
100℃
97.5-106
Oliva-Teles et al.,
2002
1-10l
0.319
0.957
30 min
Room
temperature
>95
This paper
0.3-25
0.2
0.5
30min
Room
temperature
70-78
Y = 750.03X + 8.1829
R² = 1
0
5000
10000
15000
20000
0 10 20 30
Area
FA standard solution(µg/mL)
A Rapid Determination of Free Formaldehyde Content in Marine Products
49

3.2.3 FA Content in Seafood Detecting by
Above Method
Table 3 showed comparison of free FA content in
seafood by two methods. The method in this paper
showed lower concentration of derivative and less
detecting time, the reaction was under room
temperature, which made it easier to operate. Anova
(P=0.923>0.05) was analyzed by SPSS, it showed no
difference between the two methods. The FA content
of 14 kinds of sea products was detected, the results
showed that FA content of various seafood was
different. The FA content of tuna, cod, Surf Calm and
cuttlefish was higher all above 40 mg/kg. Meanwhile
this paper studied free FA in 28 types of packaged
squid products, results showed that the average FA
content was 14.7 mg/kg, ranging from 2.10-61.8
mg/kg.
Table 3: The comparison of free FA content in seafood by
two methods.
FA content
[mg/kg]
Suggested
method
literature
method
Penaeus
vannamei Boone
10.8
11.4
Salmon
25.7
29.6
Tuna
51.5
56.3
Cod
55.7
53.8
Surf Calm
71.4
68.2
Cuttlefish
41.8
44.2
Octopus
33.7
37.2
Peru Squid
7.63
7.50
Todarodes
Pacificus
27.2
26.5
Uroteuthis
edulis
Not detected
Not detected
Loligo
Chinensis
Not detected
Not detected
Loligo
Duvaucelii
6.10
6.33
4 CONCLUSIONS
Based on the previous research, this paper improved
the determination of free FA in aquatic products by
HPLC. The free FA in Marine products was fully
reacted with the derivative reagent at room
temperature for 30 min, showing a good linear
relationship, the reactant was stable for 24 h, and the
LOQ was 0.5 mg/L. This method showed no
significant difference comparing with the old method,
while this method was simpler and easier to operate
and was suitable for the determination of free FA
content in all kinds of Marine products.
ACKNOWLEDGEMENTS
This study was supported by research grants:
Identification and control technology of potential
hazard factors in pelagic polar catches
(2017YFC1600706); Central Public-Interest
Scientific Institution Basal Research Fund
(2015T08).
REFERENCES
Bechmann, I. E., 1998. J. Lebensm Wiss Technol., 31: 449
Chen, M. N., Li, M., Yao, J., et al, 2018. Shandong Chem.
Ind., 47: 64.
Chen, S., Zhu, J. L., Pan, W. C., 2017. Mod. Food Sci.
Technol., 33 (3): 116.
European Food Safety Authority, 2006. EFSA J, 415: 1
Huang, Q. J., Long. X., Chen, H., 2017. Mod. Food, 18 (2):
6.
IARC, 2004. Monographs on the evaluation of carcinogenic
risks to humans. Lyon, France: International Agency
for Research on Cancer: 88.
Liteplo, R. G., Meek, M. J., 2003. Toxicol. Environ. Health
B, 6: 85.
Lv, C. H., Chen, X. M., Shi, Y. Z., et al, 2010. Chin. J.
Chromatogr., 28 (10): 940.
Ma, Y. J., Zhao, C., Zhan, Y. S., et al, 2015. Sep. Sci., 38:
1388.
MINSAN-telegram no. 703/3266/6/1377 08.11, 1985.
Noda, T., Takahashi, A., Kondo, N., 2011. Biochem.
Biophys. Res. Commun., 404: 206.
Oliva-Teles, M. T., Paiga, P., Delerue-Matos, C. M., et al,
2002. Anal. Chim. Acta., 467: 97.
Rehbein, H., Schmidt, T., 1996. Inf. Fischwirtsch, 43: 37
Shao, S. P., Xiang, D. P., Li, H. B., et al, 2015. Food Sci.,
36 (16): 241.
Tang, X. J., Bai, Y., Duong, A., 2009. Environ. Int., 35:
1210.
Thrasher, J., Kilburn, K., 2001. Arch. Environ. Health,
2001, 56: 300.
Treezl, L., Csiba, A., Juhasz, S., Szentgyorgyi, M., et al,
1997. Z. Lebensm. Unters. Forsch. A, 205: 300.
Yasri, N. G., Seddik, H., Mosallb, M. A, 2015. Arabian. J.
Chem., 8: 487.
Yeh, T. S., Lin, T., Chen, C. C., et al, 2013. J. Food Drug.
Anal., 21: 190.
Zhang, X., Han, F., Kong, C., et al, 2018. Qual. Saf. Agro-
Prod., 2: 90.
Zhang, X., Yang, G. X., Han, F., et al, 2018. J. Food Saf.
Qual., 9 (4): 864.
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
50
