
Combination of Intravenous Methylprednisolone and
Cyclophosphamide Pulse Therapy Weekly in Severe Autoimmune
Bullous Disease: A Preliminary Report
Jesslyn Amelia
1
, Prasta Bayu Putra
1
, Yohanes Widodo Wirohadidjojo
1
, Sunardi Radiono
1
1
Department of Dermatology and Venereology Faculty of Medicine, Gadjah Mada University / Dr. Sardjito General
Hospital, Yogyakarta, Indonesia
Keywords: pemphigus, bullous pemphigoid, cyclophosphamide, methylprednisolone, pulse therapy.
Abstract: Pemphigus vulgaris (PV), pemphigus foliaceus (PF), and bullous pemphigoid (BP) are amongst autoimmune
bullous skin diseases that are characterized by bullae formation at different levels of the skin. The mainstay
treatment is still by systemic corticosteroids, but the outcome is not satisfying with many side effects and
frequent relapse. One of the drugs that can be used as steroid-sparing agent is cyclophosphamide. In this
report, we evaluate the therapy outcome of intravenous methylprednisolone and cyclophosphamide pulsed
therapy (MCP) weekly in 6 patients with severe PV, PF, and BP who have completed six MCP. The severity
of the disease was measured using Pemphigus Disease Area Index (PDAI) for pemphigus and Bullous
Pemphigoid Disease Area Index (PBDAI) for bullous pemphigoid. The aim of this report is to evaluate the
therapy response and the side effects of this regimen therapy. After being given six MCP, four patients showed
excellent response therapy, one patient showed a good response therapy, while one patient showed a poor
response therapy. MCP might be an effective treatment for severe pemphigus and BP but the side effects
should be closely monitored. Further long follow up is needed to see the possibilities of relapse and the safety
of this regimen therapy to be used in larger sample population.
1 INTRODUCTION
Pemphigus is a rare autoimmune bullous skin
disease involving the skin and mucosa that is
characterized with intraepidermal bullae formation.
Pemphigus vulgaris (PV) is the most frequent type of
pemphigus, accounting for about 70% of cases, while
pemphigus foliaceus accounts for about 20%-30% of
cases (Joly, 2011). Bullous pemphigoid is an
autoimmune subepidermal blistering disease with
incidence is estimated to be between 6 and 20 new
cases per million people (Bernard, 2017). There were
27 cases of pemphigus vulgaris, 8 cases of pemphigus
foliaceus, and 9 cases of bullous pemphigoid
hospitalized in Dr. Sardjito General Hospital from
January 2015 to December 2017.
The mainstay therapy of PV, PF, and BP have
been systemic corticosteroids, which has reduced the
mortality rate from 75% to less than 10% since their
introduction in the 1950s. However, because of the
chronicity of the disease, prolonged therapy is
necessary, leading to the development of a wide
spectrum of corticosteroid related side effects. Thus,
mortality today are caused by treatment complication
of corticosteroids (Atzmony et al, 2015). Because of
the side effects associated with long-term systemic
corticosteroids, a lot of recent research has been
directed at finding the optimal steroid-sparing agent
(Daniel, 2014).
One of the drugs that can be used as a steroid-
sparing agent is cyclophosphamide. There was an
evidence of a steroid-sparing benefit for
cyclophosphamide compared with glucocorticoid
alone (Atzmony et al, 2015). In 1984, Parischa and
Ramji introduced intravenous corticosteroid and
cyclophosphamide pulse therapy monthly with 50 mg
cyclophosphamide orally given daily that is known to
have less side effects compared to corticosteroid
group alone and have longer remission period (Gupta,
2015),(Saha, 2010). Because cyclophosphamide oral
regimen was unavailable widely in Indonesia, we
modified this regimen into intravenous
methylprednisolone and cyclophosphamide pulse
therapy weekly with oral corticosteroid between the
514
Amelia, J., Putra, P., Wirohadidjojo, Y. and Radiono, S.
Combination of Intravenous Methylprednisolone and Cyclophosphamide Pulse Therapy Weekly in Severe Autoimmune Bullous Disease: A Preliminary Report.
DOI: 10.5220/0008161005140518
In Proceedings of the 23rd Regional Conference of Dermatology (RCD 2018), pages 514-518
ISBN: 978-989-758-494-7
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
pulse given twice per week. In this report, we evaluate
the therapy outcome of MCP weekly in 6 patients
with severe PV, PF, and BP who have completed six
pulsed therapy. The severity of the disease was
measured using Pemphigus Disease Area Index
(PDAI) for pemphigus and Bullous Pemphigoid
Disease Area Index (PBDAI) for bullous pemphigoid
(Rahbar, 2014), (Daniel, 2012), (Fuertes, 2014). The
aim of this report is to evaluate the therapy response
and the side effects of this regimen therapy.
2 CASE
Six patients were included in this report. It
consisted of one case of PF, one case of BP, and four
cases of PV. All patients had already undergone
various therapies previously. The baseline
characteristics of each patient are described in table
1.
All patients had undergone biopsy and direct
immunofluorescent before starting MCP to ensure the
diagnosis of PV, PF, and BP. An informed consent
about the benefit, risk, and possible complication that
might happen was explained to the patient and their
family, and they were asked to sign an informed
consent form. Before starting MCP, evaluation of
electrocardiogram and laboratory test that consisted
of complete blood test, electrolytes, blood glucose,
albumin, renal and liver function test were done for
these patients. Contraindications include
hypersensitivity to this regimen, pregnancy and
lactation, altered renal and hepar function, leucopenia
(leucocyte < 3000/L), thrombocytopenia
(thrombocyte < 100000/L), and urine erythrocyte
sediment > 10/L. Methylprednisolone 250 mg
intravenous injection was given in the morning.
Premedication of cyclophosphamide that consisted of
10 mg diphenhidramine intravenous injection, 8 mg
ondansetron intravenous injection, and 100 mg of 2-
mercaptoethanesulfonate natrium (mesna) in 50 ml
NaCl 0.9% were given within 15 minutes. Then 500
mg of cyclophosphamide in 250 ml NaCl 0.9% was
given within 1-2 hours. After infusion of
cyclophosphamide was finished, 100 mg mesna in 50
ml NaCl 0.9% was given three times with interval of
three hours. Vital signs were assessed during and after
cyclophosphamide infusion. Evaluation of
electrocardiogram and urinalysis were done after the
MCP. This MCP regimen was repeated once weekly
for six consecutive weeks with methylprednisolone
varying from 32 mg to 64 mg given orally, twice a
week.
The severity of the disease was measured at
every visit using PDAI for patients with PV/PF, and
BPDAI for patient with BP. The score can be seen in
table 2 below.
Side effects were found in 2 patients. One patient
developed furuncles after the fourth injection of MCP
and was later healed with tetracycline 500 mg, four
times orally. One patient had nausea and vomiting
after three injection of MCP. Because of the severe
nausea and vomiting, one patient had the MCP given
every two weeks to minimize the side effects. Neither
of these patients developed severe hematologic
deprivation nor renal and liver failure.
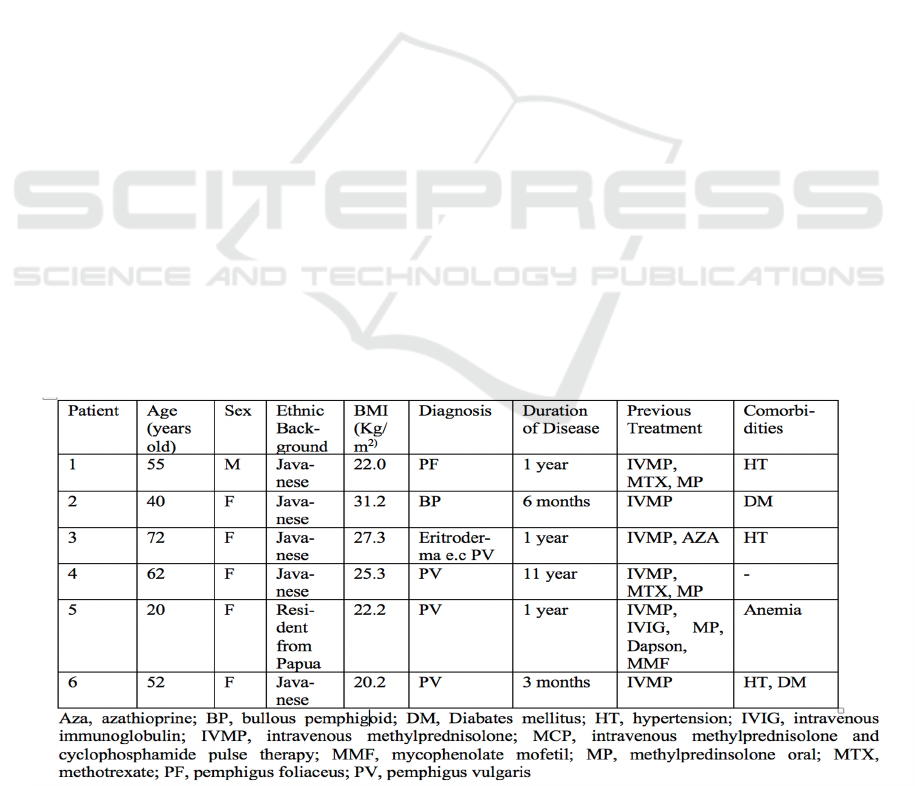
Table 1. Baseline Characteristics of Patients Before Treatment with MCP.
Combination of Intravenous Methylprednisolone and Cyclophosphamide Pulse Therapy Weekly in Severe Autoimmune Bullous Disease: A
Preliminary Report
515
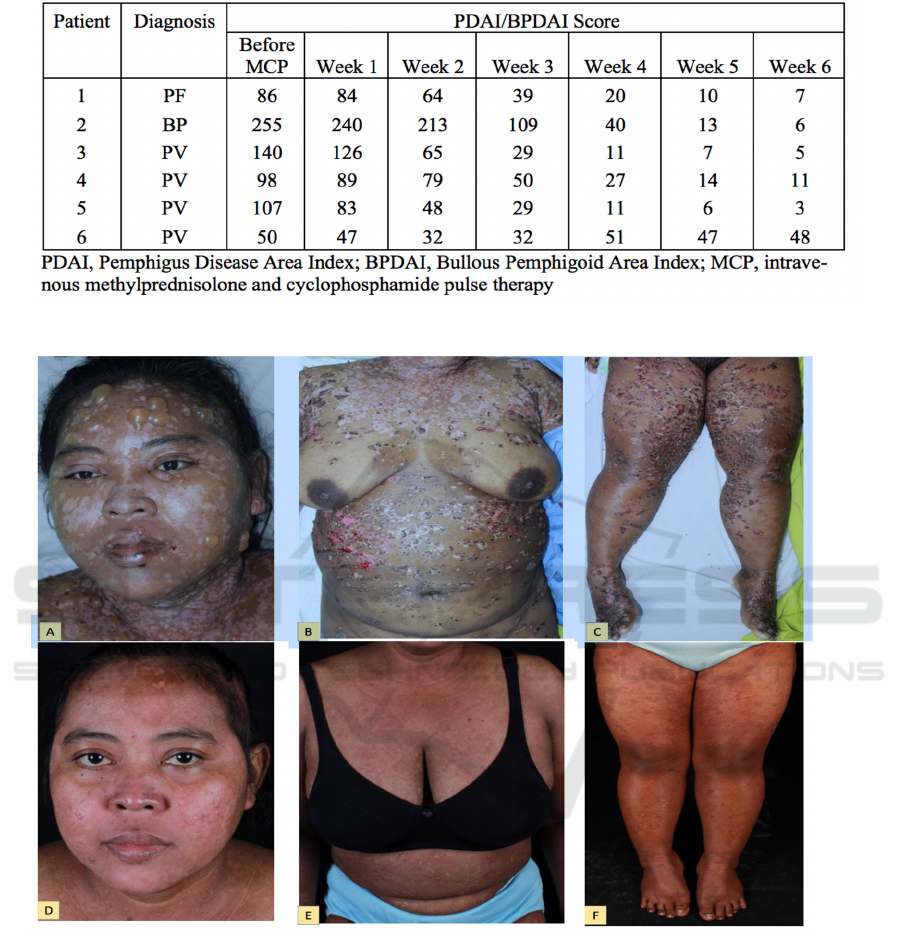
Table 2. PDAI and BPDAI Score
Figure 1. A 40 year old bullous pemphigoid patient. A-C: Pictures before starting MCP therapy; D-F: Pictures after given six
MCP therapy.
3 DISCUSSION
Cyclophosphamide is a nitrogen mustard
compound. After hepatic metabolization, it acts as an
actively alkylating agent, thus causing cross-linking
of DNA. While the half-life of the unmetabolized
substances is only short (4-6.5 hours), its metabolites
have a longer half-life. Cyclophosphamide
suppresses B and T lymphocyte responses. The
lymphocyte nadir (maximum reduction) after pulse
therapy is reached after 8-15 days. A return to
previous levels should be reached after 28 days. The
active metabolites are eliminated via the kidneys.
They are bladder toxic and may cause hemorrhagic
cystitis if hydration is insufficient. A fluid intake >
1.5 liters should be observed. The simultaneous
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
516
administration of 2-mercaptoethansulfonat-natrium
(mesna) dose adapted is definitely to be considered
for high-dose and pulse therapy. Evaluation of
complete blood count, renal and liver function test,
electrolytes, as well as urinalysis should be performed
to closely monitor the side effects that might
happened (Eming, 2015), (Shimizu, 2014).
The side effects that were happened in this report
are furuncles in one patient after the fourth MCP that
was healed after administration of systemic
antibiotics, and nausea/vomiting in one patient after
the third MCP. These side effects are common as
reported before in the previous study. Patients with
MCP therapy are more susceptible to infections,
especially when the skin and/or mucosa are eroded.
Nausea and vomiting are the most common
gastrointestinal side effects that occurred in MCP
patients (Gupta, 2015),(Saha, 2010). Any other side
effects like hematological abnormalities
(thrombocytopenia, leucopenia), electrolyte
imbalance, and signs of bladder toxicities were not
found in these patients receiving MCP.
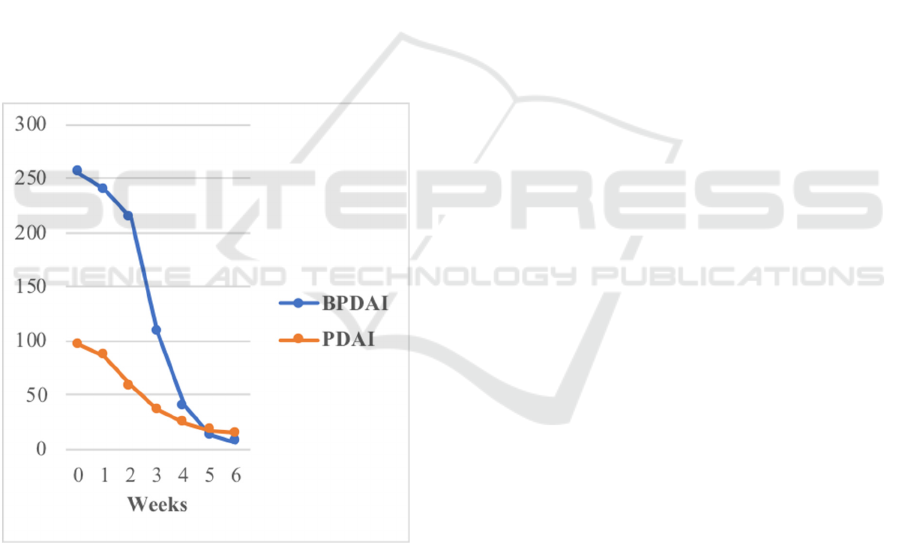
Figure 2. The Average of PDAI and BPDAI Score.
We used PDAI and BPDAI score as the disease
severity measurement of this report because it had the
highest validity (Rahbar, 2014), (Fuertes, 2014). The
PDAI score can be classified as mild (score 0-8),
moderate (score 9-24), and severe (score > 25)
(Shimizu, 2014). At the beginning of this study all
patients had severe disease. After being given six
MCP, four patients showed excellent response
therapy with PDAI/BPDAI score < 8, one patient
showed a good response with PDAI score classified
as moderate, while one patient showed a poor
response therapy. The patient with poor response to
the therapy might be caused by uncontrolled diabetes
mellitus. Although the patient had already been given
subcutaneous insulin daily, the glycemic control was
still poor because of unhealthy diet of this patient.
4 CONCLUSIONS
After being given six MCP, four patients showed
excellent response therapy, one patient showed a
good response therapy, while one patient showed a
poor response therapy. MCP might be an effective
treatment for severe pemphigus and BP but the side
effects should be closely monitored. Further long
follow up is needed to see the possibilities of relapse
and the safety of this regimen therapy to be used in
larger sample population.
REFERENCES
Atzmony, L., Hodak, E., Leshem, Y. A., Rosenbaum, O.,
Gdalevich, M., Anhalt, G. J., & Mimouni, D., 2015. The
role of adjuvant therapy in pemphigus: a systematic
review and meta-analysis. Journal of the American
Academy of Dermatology, 73(2), pp. 264-271.
Bernard, P., & Antonicelli, F., 2017. Bullous pemphigoid:
a review of its diagnosis, associations and treatment.
American journal of clinical dermatology, 18(4), pp.
513-528.
Daniel, B., & Murrell, D., 2014. Management of
pemphigus. F1000Prime Rep, 6(5), 6–9.
Daniel, B. S., Hertl, M., Werth, V. P., Eming, R., & Murrell,
D. F., 2012. Severity score indexes for blistering
diseases. Clinics in dermatology, 30(1), pp. 108-113.
Eming, R., Sticherling, M., Hofmann, S. C., Hunzelmann,
N., Kern, J. S., Kramer, H., ... & Hertl, M., 2015. S2k
guidelines for the treatment of pemphigus
vulgaris/foliaceus and bullous pemphigoid. JDDG:
Journal der Deutschen Dermatologischen Gesellschaft,
13(8), pp. 833-845.
De Vega, I. F., Iranzo-Fernández, P., & Mascaró-Galy, J.
M., 2014. Bullous pemphigoid: clinical practice
guidelines. Actas Dermo-Sifiliográficas (English
Edition), 105(4), pp. 328-346.
Gupta, R., Gupta, S., & Gupta, A., 2015. Efficacy and
safety of dexamethasone cyclophosphamide pulse
therapy in the treatment of pemphigus – An open
randomized controlled study. Apollo Med, 12(4), pp.
243–7.
Hertl, M., Jedlickova, H., Karpati, S., Marinovic, B., Uzun,
S., Yayli, S., ... & Joly, P., 2015. Pemphigus. S2
Guideline for diagnosis and treatment–guided by the
European Dermatology Forum (EDF) in cooperation
with the European Academy of Dermatology and
Combination of Intravenous Methylprednisolone and Cyclophosphamide Pulse Therapy Weekly in Severe Autoimmune Bullous Disease: A
Preliminary Report
517

Venereology (EADV). Journal of the European
Academy of Dermatology and Venereology, 29(3),
405-414.
Joly, P., & Litrowski, N., 2011. Pemphigus group (vulgaris,
vegetans, foliaceus, herpetiformis, brasiliensis). Clinics
in dermatology, 29(4), pp. 432-436.
Rahbar, Z., Daneshpazhooh, M., Mirshams-Shahshahani,
M., Esmaili, N., Heidari, K., Aghazadeh, N., ... &
Chams-Davatchi, C., 2014. Pemphigus disease activity
measurements: pemphigus disease area index,
autoimmune bullous skin disorder intensity score, and
pemphigus vulgaris activity score. JAMA dermatology,
150(3), pp. 266-272.
Saha, M., Powell, A. M., Bhogal, B., Black, M. M., &
Groves, R. W., 2010. Pulsed intravenous
cyclophosphamide and methylprednisolone therapy in
refractory pemphigus. British Journal of Dermatology,
162(4), pp. 790-797.
Shimizu, T., Takebayashi, T., Sato, Y., Niizeki, H.,
Aoyama, Y., Kitajima, Y., ... & Amagai, M., 2014.
Grading criteria for disease severity by pemphigus
disease area index. The Journal of dermatology, 41(11),
969-973.
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
518
