
Secukinumab for Psoriasis Treatment: A Case Series
Nopriyati, Radema Maradong
Department of Dermatology and Venereology Faculty of Medicine Sriwijaya University/Dr. Moh. Hoesin General Hospital
Palembang, Indonesia
Keywords: case series, psoriasis, secukinumab, PASI, BSA.
Abstract: Psoriasis is a chronic inflammatory disease of the skin and nails, is characterized by erythematous plaques
with silvery scale or pustules lesion for psoriasis pustular. Patients can suffer for psoriasis arthritis after the
moderate to severe psoriasis occurred. Methotrexate remains a first line treatment for severe to moderate
psoriasis, but due to the limitation of this medication, we decided to use secukinumab. We reported 2
patients. First case is a patient of psoriasis vulgaris with BSA of 20% and PASI 14.1 and history of
cyclosporine A treatment for 3 months, the second case is a patient of psoriasis pustular with BSA of 22%
and PASI 6 and history of methylprednisolone treatment for 6 months. First patient treated with 150 mg of
secukinumab and the second patient treated with 300 mg of secukinumab. The result after 16 weeks
treatment was satisfied which BSA and PASI was decreased. The efficacy of secukinumab should be
considered for the treatment of moderate psoriasis.
1 INTRODUCTION
Psoriasis is a chronic immune mediated disease that
affects 2-3% of the world population and is
characterized by erythematous plaques with silvery
scale (Gudjonsson & Elder, 2012).
Various forms
such chronic plaques, is most common in 90%
patients, other variant is pustular (Gudjonsson &
Elder, 2012; Yeung & Valbuena, 2016).
Psoriasis
arthritis (PsA) occur in around 20% to 30% of
patients suffering from moderate to severe psoriasis
(Gudjonsson & Elder, 2012; Yeung & Valbuena,
2016). Pathogenesis of psoriasis related to T helper
(Th)-17 and Interleukin (IL)-17, so that the
treatment of psoriasis often requires systemic
therapy, including biologic agent (Langley et al.,
2014; Becker & Jooo, 2018). Methotrexate (MTX) is
a first line choice for moderate to severe psoriasis
(Gudjonsson & Elder, 2012). Mechanism of action
MTX is blocking dihydrofolate reductase, leading to
inhibiton of purine and pyrimidine synthesis
(Gudjonsson & Elder, 2012). Due to the limitation
of MTX we chose cyclosporine A as a second line
treatment for psoriasis. Mechanism of action
cyclosporine A is binding cyclophilin and the
resulting complex blocks calcineurin, reducing the
effect of the T cells, resulting in inhibiton of IL-2
and other cytokines (Gudjonsson & Elder, 2012).
Some patients who treated by cyclosporine A
experienced high blood pressure, then we tried
another treatment.
Secukinumab is a newly approved IL-17A
monoclonal antibody. Mechanism of action is
selectively binds to the IL-17A cytokine and inhibits
its interaction with the IL-17 receptor, and also
reduce the epidermal neutrophils levels (Fala &
Writer, 2016). The administration therapy by
injection weekly at weeks 0,1,2,3 followed by
monthly maintenance dose starting at week 4
(Beecker & Joo, 2018). The recommended dose is
300 mg but for some patients, a dose of 150 mg may
be acceptable (Fala & Writer, 2016). Common
adverse effects of secukinumab are upper respiratory
tract infections, headache, diarrhea, nausea, and
neutropenia (Roman et al., 2015).
Follow up of patients in this study based on
evaluation of psoriasis area and severity index
(PASI) (Langley et al., 2014). Here we report a
cases of psoriasis vulgaris and psoriasis pustular
treated by secukinumab.
496
Nopriyati, . and Maradong, R.
Secukinumab for Psoriasis Treatment: A Case Series.
DOI: 10.5220/0008160604960499
In Proceedings of the 23rd Regional Conference of Dermatology (RCD 2018), pages 496-499
ISBN: 978-989-758-494-7
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
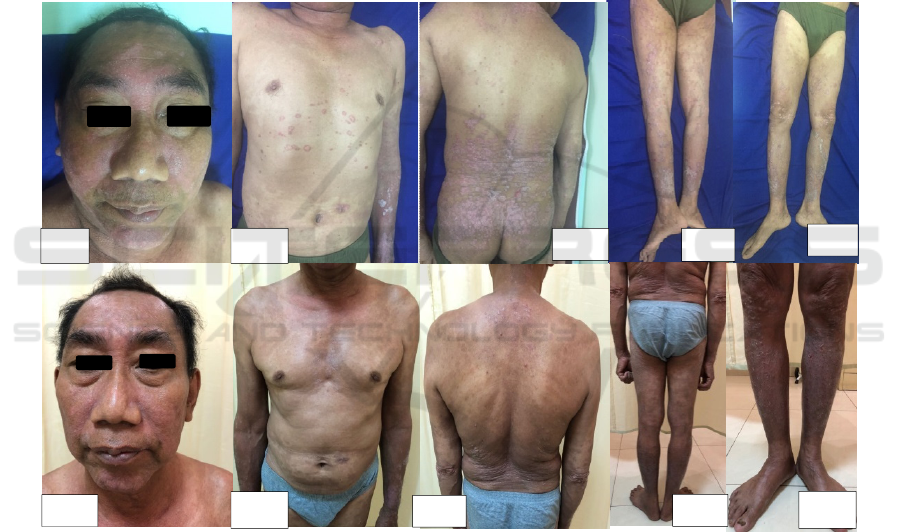
2 CASE
2.1 Case 1
A 55-years old male with erythematous plaques and
silvery scale on the scalp, face, neck, trunk and both
upper and lower extremities a year ago.
Approximately 20% of his BSA was involved, and
PASI 14.1. Laboratory examination showed elevated
of serum CRP. The patient was diagnosed with
psoriasis arthritis, he was treated with 3 months
cyclosporine but ineffective and suffered for
hypertension, then we decided to prescribed of 3
weeks 150 mg of secukinumab followed by 150 mg
every 4 weeks due to the patients’s financial. We
also prescribed omeprazole 20 mg/day and topical
treatment of cream urea 10% applied twice a day
and desoximethasone 0.25% cream twice daily. At
16 weeks, he almost reached PASI 75 (BSA of 9%,
PASI 5.9). He had not symptoms of adverse effects
(Figure 1)
2.2 Case 2
A 41-years old female with multiple 2 mm-3 mm
pustules on a diffused-erythematous and oedematous
base, some pustules developed “pus lakes” and the
rupture of pustules leave an erosions and crusts on
the trunk and both of upper and lower extremities,
since 3 years ago. Laboratory examination is normal.
Figure 1. (1a-1e) clinical features at baseline (1f-1j) clinical features at weeks 16.
Approximately 22% of her BSA was involved and
PASI 6. She was treated by unknown dose of
methylprednisolone injection followed by
methylprednisolone oral (unknown dose) for 6
months. She experienced moon face,gastritis and
striae on the abdominal due to side effects of
methylprednisolon. The patient was diagnosed with
psoriasis pustular and we decided to prescribed 3
weeks of 300 mg secukinumab followed by 300 mg
every 4 weeks. We also prescribed topical treatment
such as vaseline album twice a day followed by
vitamin C serum for hyperpigmentation lesion twice
a day. At 16 weeks, she achieved PASI 100 (both of
BSA and PASI are 0). She had not symptoms of
adverse effects (Figure 2)
3 DISCUSSION
Secukinumab is a human monoclonal
immunoglobulin (Ig) G antibody targeting IL-17A,
and has indication for both psoriasis and psoriatic
arthritis (Beecker & Joo, 2018).
The recommended
dose for adult with moderate psoriasis is 300 mg
subcutaneously at weeks 0,1,2,3 followed by 300 mg
1a
1e
1d 1c
1b
1f
1g 1j
1i
1h
Secukinumab for Psoriasis Treatment: A Case Series
497
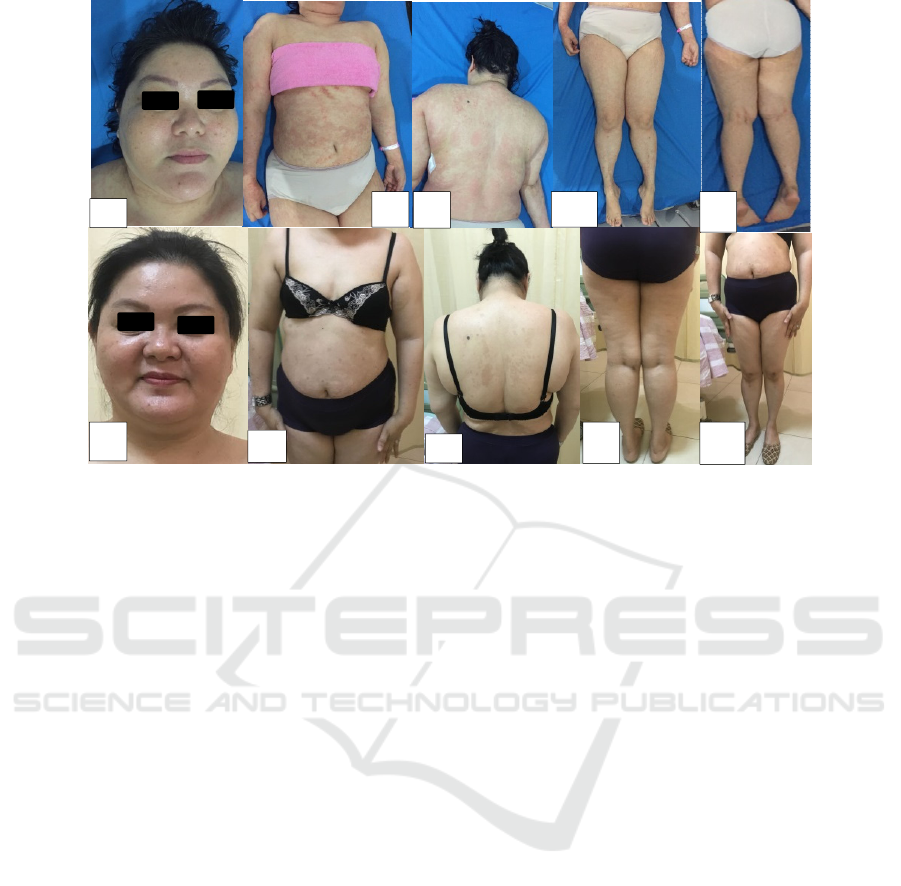
Figure 2. (2a-2e) clinical features at baseline (2f-2j) clinical features at weeks 16.
every 4 weeks starting from weeks 4 (Beecker &
Joo, 2018).
In the case 1, the patient was treated by
secukinumab after ineffective-3 months-treatment
with cyclosporine, after 16 weeks of treatment with
secukinumab, he almost reached PASI 75.This result
almost similar to Ohtsuki et al.(2017) which
reported 82.4% patients showed improvement of
PASI 75 at weeks 16 with 300 mg of secukinumab
after ineffective treatment of 12 weeks of
cyclosporine.
In this case, PASI 75 achieved at
weeks 16 may be related to dose of secukinumab.
In the case 2, the patient diagnosed with psoriasis
pustular, had a history of methylprednisolone
treatment for 6 months (unknown dose) and suffered
for a moon face and gastritis. She treated by 300 mg
of secukinumab showed an improvement of PASI
100 in weeks 16. Our result is similar with CLEAR
study that reported PASI 100 achieved at weeks 16
with secukinumab 300 mg (Thaci et al., 2015).
The patients had not reported any side effect of
secukinumab such as respiratory tract infection,
headache, nausea or neutropenia. Some study
reported the common adverse effects of secukinmab
such as nasopharyngitis, headache and nausea during
the 12 weeks induction and the entire treatment
(Roman et al., 2015). We prescribed the patients
with omeprazole to reduce the adverse effect.
Both of patients have an improvement in PASI at
weeks 16, but 300 mg of secukinumab seems more
superior to reduce PASI. Some study reported
efficacy of secukinumab 300 mg and 150 mg
compare to placebo showed a greater benefit with
achieving PASI 75 at weeks 12 than placebo (Xiong
et al., 2015).
Ohtsuki et al. (2014) reported PASI 90
achieved in 86.2% patients treated by secukinumab
150 mg compare to secukinumab 300 mg at weeks
12 but the meta analysis of four references study
reported the secukinumab 300 mg had more benefit
than the secukinumab 150 mg to achieve PASI 75
(Xiong et al., 2015).
4 CONCLUSION
The efficacy of secukinumab for treating psoriasis
vulgaris and psoriasis pustular should be considered.
There is no report about adverse effect in this study.
Clinical improvement of PASI 75 and PASI 100 can
be achieved within 12-16 weeks of treatment.
REFERENCES
Beecker, J., Joo, J. 2018. Treatment of Moderate to Severe
Psoriasis with High Dose (450-mg) Secukinumab:
Case Reports of Off Label Use. 22(1):86-88
Fala L, Writer M. Consentyx (Secukinnumab): First IL-
17A Antagonist Receives FDA Approval for Moderate
to Severe Plaque Psoriasis. Am Health Drug Benefits.
2016;9:60-63
2a
2b
2c
2d
2f
2g
2h
2i
2j
2e
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
498

Gudjonsson, J.E., Elder, J.T. Psoriasis. 2012. In: Wolff K,
Goldsmith LA, Katz SI, Gilchrest BA, Paller AS,
Lefel DJ, editors. Fitzpatrick’s Dermatology in
General Medicine. 8
th
ed. New York: McGraw Hill.
p.197-231.
Langley, R.G., Elewski, B.E., Lebwohl, M., Reich, K.,
Griffiths, C.E.M., Papp, K., Puig, L., Nakagawa, H.,
Spelman, L., Sigurgeirsson, B., Rivas, E., Tsai, T.-F.,
Wasel, N., Tyring, S., Salko, T., Hampele, I., Notter,
M., Karpov, A., Helou, S., Papavassilis, C., 2014.
Secukinumab in plaque psoriasis--results of two phase
3 trials. The New England journal of medicine 371,
326–338. doi:10.1056/NEJMoa1314258
Ohtsuki, M., Morita, A., Abe, M., Takahashi, H., Seko, N.,
Karpov, A., Shima, T., Papavassilis, C., Nakagawa,
H., 2014. Secukinumab efficacy and safety in
Japanese patients with moderate-to-severe plaque
psoriasis: Subanalysis from ERASURE, a randomized,
placebo-controlled, phase 3 study. The Journal of
Dermatology 41, 1039–1046. doi:10.1111/1346-
8138.12668
Ohtsuki, M., Morita, A., Igarashi, A., Imafuku, S., Tada,
Y., Fujita, H., Fujishige, A., Yamaguchi, M., Teshima,
R., Tani, Y., Nakagawa, H., 2017. Secukinumab
improves psoriasis symptoms in patients with
inadequate response to cyclosporine A: A prospective
study to evaluate direct switch. Journal of
Dermatology 44, 1105–1111. doi:10.1111/1346-
8138.13911
Roman, M., Madkan, V.K., Chiu, M.W., 2015. Profile of
secukinumab in the treatment of psoriasis: Current
perspectives. Therapeutics and Clinical Risk
Management. doi:10.2147/TCRM.S79053
Thaçi, D., Blauvelt, A., Reich, K., Tsai, T.F., Vanaclocha,
F., Kingo, K., Ziv, M., Pinter, A., Hugot, S., You, R.,
Milutinovic, M., 2015. Secukinumab is superior to
ustekinumab in clearing skin of subjects with
moderate to severe plaque psoriasis: CLEAR, a
randomized controlled trial. Journal of the American
Academy of Dermatology 73, 400–409.
doi:10.1016/j.jaad.2015.05.013
Xiong, H.-Z., Gu, J.-Y., He, Z.-G., Chen, W.-J., Zhang,
X., Wang, J.-Y., Shi, Y.-L., 2015. Efficacy and safety
of secukinumab in the treatment of moderate to severe
plaque psoriasis: a meta-analysis of randomized
controlled trials. International journal of clinical and
experimental medicine 8, 3156–72.
Yeung J, Valbuena V. Successful Use of Secukinumab in
Pustular Psoriasis. JAAD Case Rep. 2016;2(6):470-2
Secukinumab for Psoriasis Treatment: A Case Series
499
