
Generalized Primary Anetoderma: A Rare Case
Dina Fatmasari, Reti Hindritiani, Kartika Ruchiatan, R. M. Rendy Ariezal E.
Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran - Dr. Hasan Sadikin Hospital,
Bandung 40161 Indonesia
Keywords: Anetoderma, autoimmunity, generalized, primary
Abstract: Anetoderma is a rare elastolytic disorder characterized by localized areas of slacks skins, that are reflection
of a marked reduction or absence of dermal elastic fibers, that can appear as round or oval atrophic lesions,
and wrinkling or sac-like protrutions. The predilection of lesions are on the trunk, thigh, and upper arms,
and rarely occur on generalized distribution,. Primary anetoderma occur when there is no underlying
cutaneous diseases, but could be associated with autoimmune diseases such as antiphospholipid syndrome
and autoimmune connective tissue diseases. The aim of this report was to present a rare case of generalized
primary anetoderma. A 19-year-old female had asymptomatic, multiple, small, circumscribed, round and
oval atrophic lesions and sac-like protrution on almost all part of the body since 6 years ago without
preceding cutaneous diseases. Histopathological examination with Verhoeff-Van Gieson elastin stain
revealed fragmented and decreased of elastic fibers from superficial dermis until mid-dermis, and loss of
elastic fibers in mid-dermis that confirmed the diagnosis of anetoderma. Laboratory test for autoimmune
connective tissue diseases and antipospholipid syndrome were negative. Anetoderma is a benign disease,
but can remain active for many years resulting in considerable aesthetic deformity, moreover in generalized
distribution. Although no autoimmunologic abnormalities, long term follow-up is mandatory as an early
warning signs of future symptoms of autoimmunity.
1 INTRODUCTION
Anetoderma is an uncommon disease characterized
by focal loss of elastic tissue, resulting well-
circumscribed areas of atrophic skin or sac-like
protrutions (Bilen et al., 2003). The pathogenesis of
anetoderma is still unknown (Staiger et al., 2008;
Maari and Powell, 2012). Due to its low frequency,
only isolated reports and series with few cases have
been published (Staiger et al., 2008). The
characteristic of lesions are circumscribed areas of
slack skin with the impression of loss dermal elastic
tissue forming round or oval atrophic lesions, and
wrinkling or sac-like protrutions (Maari and Powell,
2012). The lesions commonly occur in young adults
with the usual locations on the trunk, thighs, and
upper arms, less commonly on the neck and face and
rarely elsewhere (Burrows et al., 2010). According
to author knowledge, there are only two cases of
generalized anetoderma have been reported (Emer et
al., 2013; Inamadar et el., 2003). Anetoderma
lesions classified into primary and secondary types
(Emer et al., 2013). Primary anetoderma develops in
clinically normal skin and have been related to a
variety of pathologies, mainly autoimmune diseases
(Staiger et al., 2008). Primary anetoderma may be as
a cutaneous sign of autoimmunity, whereas
autoimmune diseases may develop later after the
onset of anetoderma (Al Buainain and Allam, 2009).
Secondary anetoderma arises on previous skin
diseases, although not always in relation to the
lesions, and could be associated with syphilis and
Human immunodeficiency virus (HIV) (Bilen et al.,
2003). There is no effective treatment for
anetoderma (Maari and Powell, 2012), the new
lesions often continue to develop for many years as
the older lesions fail to resolve (Maari and Powell,
2012). The aim of this report was to present a rare
case of generalized primary anetoderma.
2 CASE
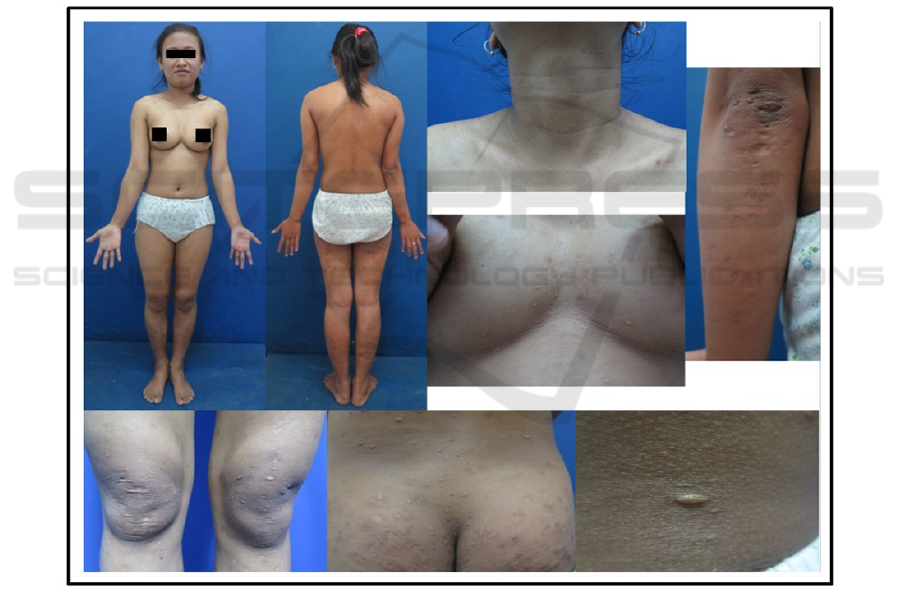
A 19-year-old female was noticed to have had
asymptomatic, multiple, small, circumscribed, round
and oval atrophic lesions and sac-like protrution on
446
Fatmasari, D., Hindritiani, R., Ruchiatan, K. and Ariezal E., R.
Generalized Primary Anetoderma: A Rare Case.
DOI: 10.5220/0008159404460450
In Proceedings of the 23rd Regional Conference of Dermatology (RCD 2018), pages 446-450
ISBN: 978-989-758-494-7
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

almost all part of the body. The lesions started on
elbows without history of skin lesion on the areas of
the lesions and spread to almost all part of the body
in 6 years duration. Skin lesions consisted of
scattered, well-circumscribed, round and oval, skin
color and white lesions, atrophic and some lesions
raised above the surrounding skin level (sac-like
protrutions). On palpation, a hernia orifice could be
fell under the finger, and the content of the hernia
sac could be pressed through this orifice, producing
the “button-hole sign”. Size of lesions are 0,5 until 2
centimeter in diameter with central protrusion, that
were distributed on almost all part of the body with
sparing in scalp, palms, and soles (figure 1). No
sensory changes associated with the lesions. She did
not have any history of medication consumption. No
family history with the same symptoms. Skin biopsy
was taken from atrophic and protruding lesions for
histopathological examination and both revealed
perivascular and periadnexal lymphocyte
inflammatory cell infiltrate (figure 2A). Verhoeff-
Van Gieson elastin stain showed fragmented and
markedly decreased of elastic fibers in the
superficial dermis until mid-dermis and loss of
elastic fibers in mid-dermis (figure 2B) supported
the diagnosis of anetoderma. Direct
immunofluorescence (DIF) did not performed.
Complete blood count, erythrocyte sedimentation
rate, and routine chemistry were normal. The patient
did not have any symptoms or show any signs of
antiphospholipid syndrome (APS) and screening for
antiphospholipid antibodies (anticardiolipin IgG and
IgM) were negative. Bleeding time and clotting time
were within normal range. Antinuclear antibody
(ANA) and ANA profile were negative. Thyroid
panel test did not performed. Venereal disease
research laboratory (VDRL) test, Treponema
pallidum haemagglutination assay (TPHA), anti-
HIV were all non-reactive.
3 DISCUSSION
The term “anetoderma” is derived from anetos, the
Greek word for slack, and derma for skin (Maari and
Powell, 2012). This disease favors young adults
between 15 (Maari and Powell, 2012) and 40 years
(Aghaei et al., 2004). and occurs more frequently in
women than men (Maari and Powell, 2012; Aghaei
et al., 2004). The characteristic lesions of
anetoderma are flaccid circumscribed areas of slack
skin that are reflection of a marked reduction or
absence of dermal elastic fiber and can appear as
depressions, wrinkling or sac-like protrusions (Maari
and Powell, 2012). The lesions appear as
circumscribed round or oval areas of the skin with
atrophic aspect (Staiger et al., 2008) and protrude
from the skin and on palpation have less resistance
than the surrounding skin, producing the “button-
hole” sign. (James et al., 2016). The lesions can vary
in number from less than five (Burrows et al., 2010)
to hundreds, and they typically measure 0,5 (Aghaei
et al., 2004) -2 cm in diameter (Maari and Powell,
2012). The color varies from skin color, blue, white
(Maari and Powell, 2012); Burrows et al., 2010), and
grey (Burrows et al., 2010). The surface skin may be
slightly shiny, white, and crinkly. The patient may
be totally asymptomatic or refer pruritus on the
lesions (Staiger et al., 2008). In our case, the patient
is a 19 years-old female, presented with
asymptomatic, multiple, small, circumscribed, round
and oval atrophic lesions and sac-like protrution on
almost all part of the body. The lesions are 0,5 until
2 centimeter in diameter with central protrusion,
skin color and white, with some had slightly shiny
and crinkly surface.
The usual locations of anetoderma are the trunk,
especially on the shoulder, the upper arms, and thigh
(Maari and Powell, 2012; Burrows et al., 2010), less
commonly on the neck and face and rarely
elsewhere. The scalp, palms, and soles are usually
spared (Burrows et al., 2010). There were only few
reported cases of anetoderma with generalized
distribution (Emer et al., 2013; Inamadar et al.,
2003). Emer et al. (2013) reported one case of
generalized anetoderma in a patients with secondary
syphilis after being treated with intravenous
penicillin. Inamadar et al. (2003) reported a case of
generalized secondary anetoderma in a patient with
HIV infection, pulmonary tuberculosis and
lepromatous leprosy. The new lesions often continue
to form for many years as the older lesions fail to
resolve (Maari and Powell, 2012. The distribution of
the lesions in this report are generalized, affecting
almost all part of the body, and sparing on scalp,
palms, and soles. The lesions started on elbows
without history of skin lesion on the areas of the
lesions and spread to almost all part of the body in 6
years duration.
The pathogenesis of anetoderma is still unknown
(Staiger et al., 2008; Maari and Powell, 2012). The
loss of dermal elastin may reflect an impaired
turnover of elastin, caused by either increased
destruction or decreased synthesis of elastic fibers.
There are a number of proposed explanations for the
focal elastin destruction such as the release of
elastase from inflammatory cells, the release of
cytokines such as interleukin-6, an increased
Generalized Primary Anetoderma: A Rare Case
447
production of progelatinases A and B, and the
phagocytosis of elastic fibers by macrophages
(Maari and Powell, 2012).
The diagnosis of anetoderma is based on an
association of clinical features, and the
histopathological examination. In the routinely
stained sections, the collagen within the dermis of
affected skin appears normal. A perivascular
inflammatory infiltrate is present that lymphocytes
were the predominant cell type. There are usually
some residual abnormal irregular and fragmented
elastic fibers. Plasma cells and histiocytes with
occasional granuloma formation can be seen (Emer
et al., 2013). The predominant abnormality is a
focal, more or less complete loss of elastic tissue in
the papillary and/or mid-reticular dermis as revealed
by elastic tissue stain such as Verhoeff-Van Gieson
stain (Staiger et al., 2008; Maari and Powell, 2012).
Direct immunofluorescence sometimes shows linear
or granular deposits of immunoglobulins and
complement along the dermal-epidermal junction or
around the dermal blood vessels in affected skin.
However, these findings are not helpful
diagnostically (Maari and Powell, 2012). In this
patient, the histopathologic examination revealed
perivascular and periadnexal lymphocyte
inflammatory cell infiltrate. Elastic stain (Verhoeff-
Van Gieson) showed fragmented and markedly
decreased of elastic fibers in the superficial dermis
until mid-dermis and loss of elastic fibers in mid-
dermis and the diagnosis was consistent with
anetoderma. We did not perform direct
immunofluorescence (DIF) examination in this
patient.
Figure 1. Multiple, small, circumscribed, round/oval, skin color and white, atrophic lesions and sac-like protrutions on
almost all part the body with “button-hole sign” on palpation.
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
448
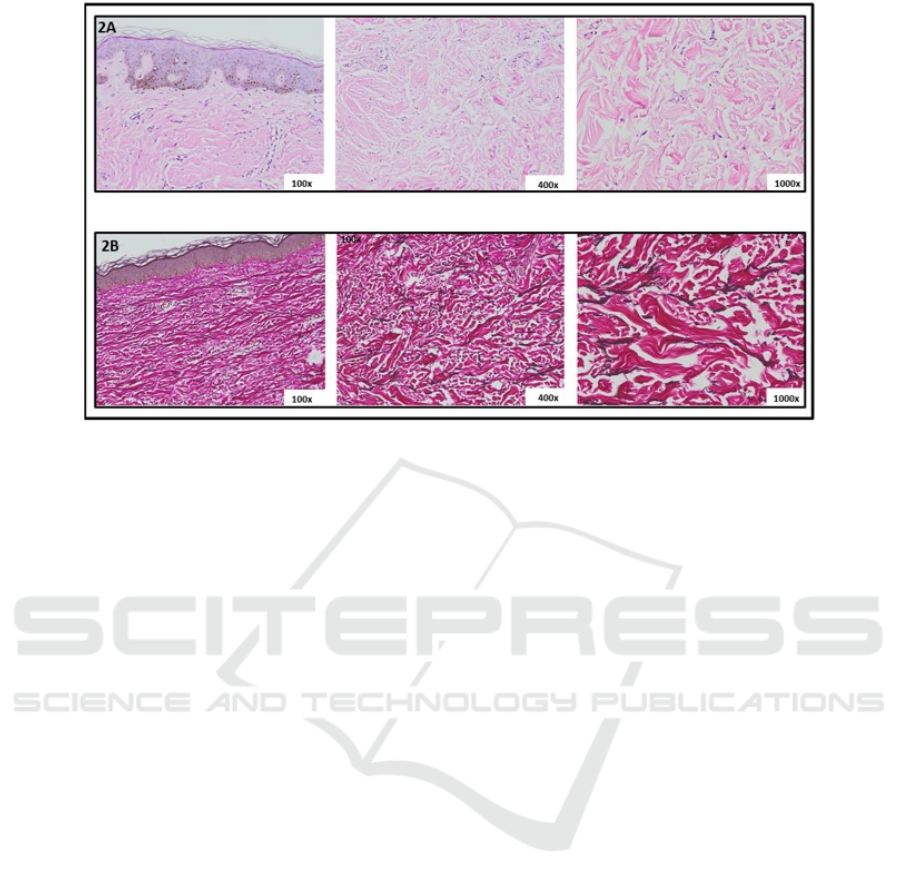
Figure 2. Histopathological examination. 2A. Hematoxilin-Eosin stain 2B. Verhoeff-Van Gieson elastin stain
Two forms of anetoderma are traditionally
distinguished, primary and secondary (Staiger et al.,
2008; Persechino et al., 2011). The lesions of
primary anetoderma occur in clinically normal skin
(Maari and Powell, 2012; Persechino et al., 2011)
although there could be an association with other
dermatological or systemic diseases or conditions,
without a well-established relationship (Persechino
et al., 2011). Some author classified primary
anetoderma into two major forms, Jadassohn-
Pellizzari type which is preceded with inflammatory
lesions, and Schweninger-Buzzi type without
preceding inflammatory lesions. This clinical
classification is primarily of historical interest, since
the two types of lesions can coexist in the same
patient and their histopathology is often the same
and not related to prognosis (Maari and Powell,
2012). In the secondary anetoderma the lesions can
occurs on the same site as another skin lesions
(Persechino et al., 2011), including acne, insect
bites, varicella, syphilis, leprosy, tuberculosis,
granuloma annulare, and urticarial pigmentosa, but
no always in relation to the lesions (Emer et al.,
2013). The diagnosis of primary anetoderma can be
established only by excluding the presence of any of
the diseases known to be associated with secondary
atrophy (Burrows et al., 2010). We diagnosed this
patient as primary anetoderma because the patient
did not noticed any signs of inflammatory lesions or
skin diseases preceded the lesions. We performed
laboratory test for syphilis and HIV infection which
could be the etiology of secondary anetoderma, and
the results were all non-reactive.
Primary anetoderma has been related to multiple
diseases. For some time, its association with
autoimmune pathologies has been highlighted,
mainly systemic lupus erythematosus and
antiphospholipid syndrome (Staiger et al., 2008).
There are some evidence to consider primary
anetoderma as a cutaneous sign of positive
antiphospholipid antibodies with or without
fulfilling the criteria antiphospholipid antibodies
syndrome (Al Buainain and Allam, 2009). Several
studies have subsequently linked anetoderma with
concurrent Grave’s disease (autoimmune
thyroiditis), autoimmune hemolysis, systemic
sclerosis, and alopecia areata (Emer et al., 2013).
Xia et al. (2016) reported a case of anetoderma in a
patient with SLE, who around the time of
anetoderma presentation was negative for
antiphospholipid antibody but who later went on to
develop antiphospholipid antibody (IgM
anticardiolipin). They suggest that anetoderma can
precede antiphospholipid antibody serologic
conversion and continued monitoring of
antiphospholipid serology regardless of initial
serology will be important. Bergman et al. (1990)
described a case of primary hypothyroidism that
developed 3 years after the onset of anetoderma.
From some literature suggested to think of primary
anetoderma as a cutaneous sign of autoimmunity and
patients should be examined and carefully tested for
autoimmune diseases, especially for
antiphospholipid antibodies, lupus erythematosus
and also thyroid antibodies. Patients should also be
followed up because associated autoimmune
Generalized Primary Anetoderma: A Rare Case
449

diseases may develop later in the course of the
disease (Al Buainain and Allam, 2009). Evaluation
for the presence of antiphospholipid antibodies
should be performed before patients with idiopathic
anetoderma are labeled as having true primary
anetoderma (Maari and Powell, 2012). Laboratory
examinations for autoimmune connective tissue
diseases and antipospholipid syndrome in this
patient were negative. There were no sign and
symptoms of autoimmunity diseases confirmed the
diagnosis of idiopathic primary anetoderma. In this
patient, we plan long-term follow-up to monitor if
there will develop any signs and laboratory
abnormalities suggest to autoimmunity diseases.
Anetoderma is a benign condition (Persechino et
al., 2011), and the disease can remain active for
many years resulting in considerable aesthetic
deformity (Zaki et al., 1994). Venencie et al. (1984)
studied 16 patients and found that no treatment was
beneficial once the depressed lesions had developed.
Various therapeutic modalities have been tried, but
have not resulted in improvement of existing
atrophic lesions, these include intralesional
injections of triamcinolone and systemic
administration of aspirin, dapsone phenytoin,
penicillin G, vitamin E, and inositol niacinate.
Surgical excision of limited lesions may be helpful,
with consideration the possibility of scars formation
(Maari and Powell, 2012).
4 CONCLUSION
Anetoderma is rarely occur in generalized
distribution. In each case of anetoderma, it is
important to determine the case of primary or
secondary, and patients should be examined,
carefully tested, and followed up for autoimmune
diseases. This is a benign condition, no effective
treatment, and the new lesions often continue to
form as the older lesions fail to resolve results in
aesthetic deformity.
REFERENCES
Aghaei S, Sodaifi M, Aslani FS, Mazharinia N. An
unusual presentation of anetoderma: a case report.
BMC Dermatol. 2004;4(1):9.
Al Buainain H, Allam M. Anetoderma: Is It a Sign of
Autoimmunity? Case Rep Dermatol. 2009;1(1):100-4.
Bergman R, Friedman‐Birnbaum R, Hazaz B, Cohen E,
Munichor M, Lichtig C. An immunofluorescence
study of primary anetoderma. Clin Exp Dermatol.
1990;15(2):124-30.
Bilen N, Bayramgürler D, Şikar A, Ercin C, Yilmaz A.
Anetoderma associated with antiphospholipid
syndrome and systemic lupus erythematosus. Lupus.
2003;12(9):714-6.
Burrows NP, Lovell CR. Anetoderma. In: Burns T,
Breathnach S, Cox N, Griffiths C, editors. Rook's
textbook of dermatology.8
th
ed. Oxford: Willey-
Blackwell; 2010. h. 45.17-45.18.
Emer J, Roberts D, Sidhu H, Phelps R, Goodheart H.
Generalized Anetoderma after intravenous penicillin
therapy for secondary syphilis in an HIV Patient. The
Journal of clinical and aesthetic dermatology.
2013;6(8):23.
Inamadar AC, Palit A, Athanikar S, Sampagavi V,
Deshmukh N. Generalized anetoderma in a patient
with HIV and dual mycobacterial infection. Lepr Rev.
2003;74(3):275-8.
James WD, Berger TG, Elston DM, Isaac MN.
Anetoderma (macular atrophy). In: James WD, Berger
TG, Elston DM, Isaac MN, editors. Andrews' diseases
of the skin clinical dermatology.12
th
ed. Philadelphia:
Elsevier-Book Aid International; 2016. p. 507.
Maari C, Powell J. Atrophies of connective tissue. In:
Bolognia JL, Jorizzo JL, Schaffer JV, Editors.
Dermatology. 3
rd
ed. New York: Elsevier Saunders;
2012. p. 1631-40.
Maari C, Powell J. Antoderma and other atrophic
disorders of the skin. In: Goldsmith LA, Katz SI,
Gilchrest BA, Paller AS, Leffel DJ, Wolff K, editors.
Fitzpatrick's dermatology in general medicine.8
th
ed.
New York: McGraw-Hill; 2012. p. 718-24.
Persechino S, Caperchi C, Cortesi G, Persechino F, Raffa
S, Pucci E, et al. Anetoderma: evidence of the
relationship with autoimmune disease and a possible
role of macrophages in the etiopathogenesis. Int J
Immunopathol Pharmacol. 2011;24(4):1075-7.
Staiger H, Saposnik M, Spiner RE, Schroh RG, Corbella
MC, Hassan ML. Primary anetoderma and
antiphospholipid antibodies. Dermatología Argentina.
2008;14(SE):2008; 14 (5): 372-378.
Venencie PY, Winkelmann R, Moore BA. Anetoderma:
clinical findings, associations, and long-term follow-
up evaluations. Arch Dermatol. 1984;120(8):1032-9
Xia FD, Hoang MP, Smith GP. Anetoderma before
development of antiphospholipid antibodies: delayed
development and monitoring of antiphospholipid
antibodies in an SLE patient presenting with
anetoderma. Dermatol Online J. 2016;23(3).
Zaki I, Scerri L, Nelson H. Primary anetoderma:
phagocytosis of elastic fibres by macrophages. Clin
Exp Dermatol. 1994;19(5):388-90.
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
450
