
Hemorrhagic Varicella in Osteosarcoma: Case Report Is It Induced
by Chemotherapy Agent or Nature of the Disease?
Benny Nelson, Matahari Arsy Harum P., Vita Siphra, Eliza Miranda, Sri Linuwih Menaldi
Department of Dermatology and Venereology, Faculty of Medicine Universitas Indonesia / Dr. Cipto Mangunkusumo
National General Hospital, Jakarta, Indonesia
Keywords: varicella, hemorrhagic, osteosarcoma, immunocompromised, thrombocytopenia
Abstract: Varicella is a result of primary varicella-zoster virus infection and can be found worldwide. Eventhough
varicella is a self-limiting disease, serious complications can occur in 2-6% cases, especially in neonatus,
adults, and immunocompromised patients. The uncommon hemorrhagic complication that occurs in these
patients could be the result of thrombocytopenia, increased capillary pressure secondary to cutaneous
hyperemia, or bacterial secondary infection. Thrombocytopenia state can be caused by the chemoterapy agent
and be worsen by the increasing antibody response to the platelets, interaction between the virus and the
platelets resulting early removal of platelets by the reticuloendothelial system, or the release of the
neuraminidase from the virus. Mortality rates of complicated varicella is 63%. We report 2 patients with
hemorrhagic varicela in osteosarcoma in the facial region and thrombocytopenia. Both patients were clinically
diagnosed with varicella and were given intravenous acyclovir 10 mg / kg bodyweight for 10 consecutive
days. During treatment, there were no new vesicles, last crop of vesicles had crusted. Thrombocytopenia state
was corrected with thrombocyte concentrate (TC) transfusion and fortunately no internal bleeding found.
1 INTRODUCTION
Varicella is primary infection of varicella-zoster virus
(VZV). Varicella cases are distributed worldwide, but
the incidence differs in climates and vaccination
status (Schmader & Oxman, 2012). The incidence is
expected to be 60 million cases every year (Gancheva
et al., 2014). Varicella is highly contagious and could
be endemic in subtropic countries and the lack of
vaccination. About 90% of varicella occurred in
children younger than 10 years old (Schmader &
Oxman, 2012). There were approximately 11.000
hospitalization and 100 deaths caused by varicella
each year before vaccination era in 2004 (Seward et
al., 2002).
Varicella patients starts to be infectious from 1-2
days before exanthem appears and for 4-5 days after
it appears. In immunocompromised patients, the
exanthem can last longer, which means the infectious
period is also prolonged. The incubation period of
varicella is 10-23 days, with the average of 14 days
(Schmader & Oxman, 2012; Marin et al., 2016). The
major route of infection is the respiratory tract and
direct contact (Schmader & Oxman, 2012;
Gancheva et al., 2014; Seward et al., 2002; Marin
et al., 2016).
Before exanthem appears, varicella is often
preceded by 2-3 days of prodromal symptoms, though
these prodromal symptoms are uncommon in young
children. After the prodromal symptoms,
erythematous macules appear firstly on the scalp and
face, then spread rapidly to the trunk. Progressively,
the macules turn into papules, vesicles, pustules, and
crusts that distributed centrally on the body
(Schmader & Oxman, 2012; Miller & Stephan, 1993).
Differential diagnosis includes vesicular viral
exanthem, insect bites, impetigo, contact dermatitis,
and disseminated herpes simplex (Schmader &
Oxman, 2012; Miller & Stephan, 1993). Varicella can
be diagnosed from the clinical appereance and
evolution of its characteristic rash, especially if
there’s a direct contact with other varicella patient 2-
3 weeks before (Schmader & Oxman, 2012). Tzanck
smear test from the base of the vesicles shows the
presence of a characteristic multinucleated giant cells
but can’t distinguish it from other viral infection
(Leung et al., 2010; Nahass et al., 1992).
Eventhough varicella is a self-limiting disease,
serious complications can occur in 2-6% cases,
especially in neonatus, adults, and
420
Nelson, B., Harum P., M., Siphra, V., Miranda, E. and Menaldi, S.
Hemorrhagic Varicella in Osteosarcoma: Case Report Is It Induced by Chemotherapy Agent or Nature of the Disease?.
DOI: 10.5220/0008158804200423
In Proceedings of the 23rd Regional Conference of Dermatology (RCD 2018), pages 420-423
ISBN: 978-989-758-494-7
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

immunocompromised patients (Schmader & Oxman,
2012, Miller et al., 1993; Bonanni, 2009). The
complications can occur before, during, or after the
presence of rashes. Most complications of varicella
can be grouped into eight major categories: (1)
bacterial superinfection; (2) herpes zoster; (3)
varicella-associated Reye’s syndrome; (4) central
nervous system; (5) pulmonary; (6) hemorrhagic; (7)
therapeutic complications or exacerbation of
underlying illnesses; and (8) immunocompromised
patients (Miller et al., 1993). Hemorrhagic
complication that occurs in varicella is frequently
associated with secondary thrombocytopenia or
secondary infection. The states of thrombocytopenia
relate to increased capillary pressure secondary to
cutaneous hyperemia causes the clinical hemorrhagic
appeareance (Charkes, 1961). Mortality rates of
complicated varicella is as high as 63% (Miller et al.,
1993).
2 CASE
We report 2 cases of hemorrhagic varicella in
osteosarcoma patients. First patient was a 16 years old
girl, presented to Pediatric Emergency Unit of Cipto
Mangunkusumo General Hospital (RSCM) with dark
vesicles and bullous on her body from 10 days before
admission. It started with 3 erythematous vesicles on
the face and high fever. Within the same day, the
vesicles spread to the other part of her body and
turned dark. New dark vesicles still appeared 1 day
before admission on the soles of the feet. She also
suffered pain sensation all over her body, which made
her uncomfortable and hard to sleep. The patient had
not given any medication before admission, except
for the paracetamol she had been taking since her first
chemotherapy.
Two weeks before the first vesicles appeared,
patient held her nephew who was having varicella.
There was no history of applying anything before
vesicles appeared. There was no history of loss of
consciousness, dispnea, cough, blurred vision, or
upper abdomen pain. The patient was diagnosed with
nasal osteosarcoma and was on the third
chemotherapy and took paracetamol regularly to
reduce the pain. The patient never had varicella and
varicella vaccination before.
From the physical examination, the vital signs
were within normal limit. Her body weight was 60 kg
and her body height was 158 cm (BMI: 24,03). We
found multiple, circumscribed, discrete hemorrhagic
vesicle-bullous and some black crusts all over her
body. There was no lymph enlargement. Laboratories
studies obtained: hemoglobin was 9,59 g/dL;
leukocyte was 2,58 x 10
3
/μL; platelet count was 17 x
10
3
/μL; and albumin was 2,23 g/dL. Other
laboratories studies were within normal limit.
The patient was diagnosed with osteosarcoma
grade IV on third cycle chemotherapy and varicella
with pancytopenia and hypoalbuminemia state. The
patient was admitted to the isolation room and given
intravenous acyclovir 600 mg every 8 hours,
intravenous ampicillin-sulbactam 2 gram every 6
hours, oral paracetamol 500 mg every 8 hours,
albumin 20% transfusion 2 x 100 mL, thrombocyte
concentrate (TC) transfusion, and high protein diet
(1,5 g/kg body weight/day).
On the fifth day of IV acyclovir, the patient was
getting better, there were no new lesions, while some
of the vesicles had already crusted. On the 10
th
day,
the patient felt so much better and was excited to get
home. There was no new lesions, fever, and pain. The
patient was then discharged and oral acyclovir was
continued until the 14
th
day.
Second
patient was a 23 years old male. The
patient was on the 5
th
day treatment in the ward by the
Internal Medicine Department, RSCM and was
consulted with hemorrhagic vesicles since 3 days ago
along with fever. The patient didn’t feel any pain or
itch sensation. The vesicles started as red patch on the
face and within 1 day, the red patch became vesicles
and spread to other part of his body.
There was no history of loss of consciousness,
dispnea, cough, blurred vision, or upper abdomen
pain. The patient never had varicella and varicella
vaccination before, nor contact with other varicella
patients. The patient was already diagnosed with
osteosarcoma on the mastoid region and was at the
second chemotherapy with anemia,
hypoalbuminemia, and thrombocytopenia states.
From the physical examination, the vital signs
were within normal limit. His body weight was 70 kg
and his body height was 168 cm (BMI: 24,80). We
found multiple, circumscribed, discrete black vesicle-
bullous on the skalp, neck, chest, stomach, back, both
arms and legs. No lymph enlargement was found.
Laboratories studies revealed: hemoglobin was 8
g/dL; leukocyte was 0,26 x 10
3
/μL; platelet count was
25 x 10
3
/μL; andn albumin was 3,21 g/dL. Other
laboratories studies were within normal limit.
We diagnosed the patient as varicella in
immunocompromised and thrombocytopenia state
and gave him intravenous acyclovir 700 mg every 8
hours, oral paracetamol 500 mg every 8 hours, oral
paracetamol 500 mg every 8 hours, intravenous
meropenem 1 g every 8 hours, TC transfusion, and
high protein diet (1,5 g/kg body weight/day). During
Hemorrhagic Varicella in Osteosarcoma: Case Report Is It Induced by Chemotherapy Agent or Nature of the Disease?
421
treatment, patient was getting better and there were
no new lesions since intravenous acyclovir had been
given. All the vesicles had already crusted on the 10
th
day of acyclovir.
3 DISCUSSION
Both patients were clinically diagnosed with varicella
because of the characteristic rash, which began as red
macules on the scalp and progressively turned into
papules, vesicles, pustules, and crusts that centrally
distributed. There were direct contacts with other
varicella patients in the first patient, which was her
nephew. The high fever and pain in the first patient
were the prodromal symptoms. Tzanck smear from
the base of vesicle might show multinucleated giant
cells in viral infection and although it’s not specific
for varicella, it might be helpful in confirming the
diagnosis of varicella (leung et al., 2010; Nahass et
al., 1992). We did not do Tzanck smear because the
diagnosis can already be made clinically. Usually the
treatment is limited to acetaminophen for fever and
pain, sometimes antipruritic for the itch, and
maintenance of general hygiene. Varicella patients
with immunocompromised are generally given
intravenous acyclovir for 10-14 days, which
dramatically reduces the risk of complications and
formation of new lesions (Miller et al., 1993). The
virus will then remain latent in sensory ganglia and
could reappears as herpes zoster in 10%-15%
individuals (Schmader & Oxman, 2012).
Coagulopathies are frequently associated with
varicella infection through a variety of etiological
mechanism. The uncommon hemorrhagic lesions in
both patients could be the result of thrombocytopenia
caused by the chemotherapy agent, increased
capillary pressure secondary to cutaneous hyperemia,
or bacterial secondary infection. Viral infection could
make the thrombocytopenia worse by several
mechanisms: an increased antibody response
(suggesting the possibility of an autoimmune
mechanism), interaction between the virus and the
platelets result in early removal of platelets by the
reticuloendothelial system, or the release of the
neuraminidase from the virus attacks sialic acid in the
platelet membrane. Patients with malignant or
progressive varicella with purpura, are unable to
terminate the viremia because of the changes in T-cell
subsets and maybe related to inadequate production
of interferon. Bleeding from the gastrointestinal tract,
genitourinary tract, and mucous membranes is
frequent and the mortality rate is greater than 70%
(Miller et al., 1993).
Fortunately both patients did not
have any evidence related to gastrointestinal or
genitourinary bleeding.
Varicella is highly contagious and could be
endemic with attack rates 60-100%,
10,11
and for that
reason, both patients were admitted to isolation room
(Varicella-Zoster Infections, 2009; Heininger et al.,
2006). The most common complication of varicella is
bacterial superinfection (Schmader & Oxman, 2012,
Miller et al., 1993; Bonanni, 2009). This
superinfection could lead to septic shock in
immunocompromised patients. Both patients were
given broad spectrum antibiotic for severe infections
(Nelson et al., 2016).
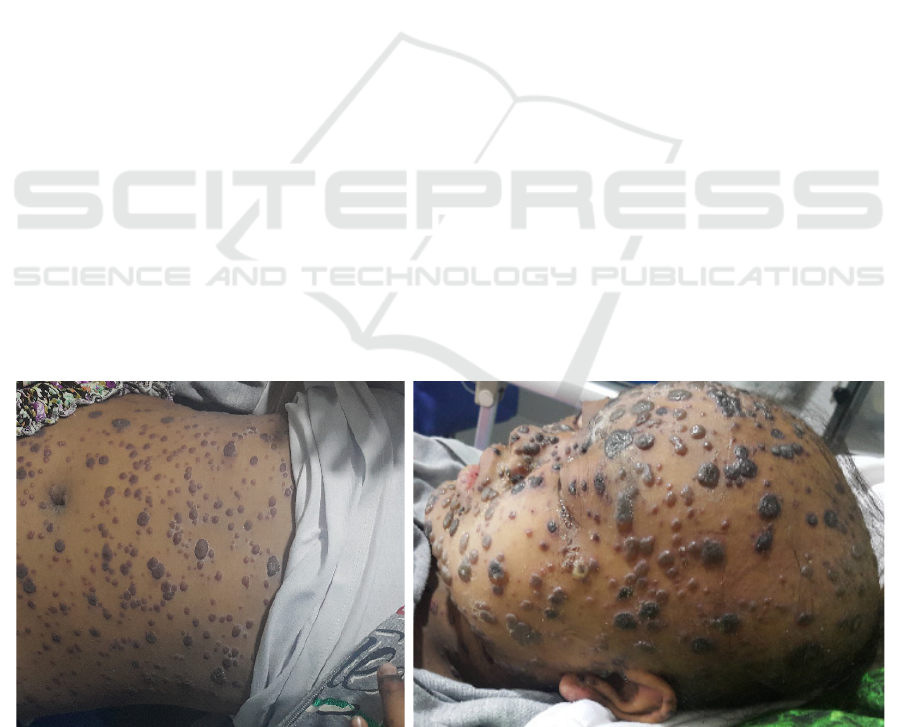
Figure 1. First patient with hemorrhagic varicella
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
422

Figure 2. Second patient with hemorrhagic varicella
4 CONCLUSION
Serious complications can occur in varicella,
especially those with immunocompromised state,
including hemorrhagic lesions. These hemorrhagic
lesions can be the result of thrombocytopenia caused
by the chemotherapy agent and increased capillary
pressure secondary to cutaneous hyperemia. The
thrombocytopenia is worsen by several mechanism,
which is the nature of the disease itself.
REFERENCES
Bonanni, P., Breuer, J., Gershon, A., Gershon, M.,
Hryniewicz, W., Papaevangelou, V., Rentier, B.,
Rümke, H., Sadzot-Delvaux, C., Senterre, J., Weil-
Olivier, C. & Wutzler, P., 2009. Varicella Vaccination
in Europe – Taking the Practical Approach, BMC
Medicine, 7(1), 26. doi: 10.1186/1741-7015-726.
Charkes, N. D., 1961. Purpuric chickenpox: report of a
case, review of the literature, and classification by
clinical features. Annals of internal medicine, 54(4), pp.
745-759.
Gancheva, G. I., Doichinova, T. G., & Lukanov, T. C.,
2014. Complications of Varicella–Report of Case with
Hemorrhagic-Necrotic Rash and Cerebellar Ataxia,
JMED Research, Article ID: 589754.
Heininger, U., & Seward, J. F., 2006. Varicella. The
Lancet, 368(9544), pp. 1365-1376.
Leung, J., Harpaz, R., Baughman, A. L., Heath, K.,
Loparev, V., Vázquez, M., Watson, B. M., & Schmid,
D. S., 2010. Evaluation of laboratory methods for
diagnosis of varicella. Clinical infectious
diseases, 51(1), pp. 23-32.
Marin, M., Marti, M., Kambhampati, A., Jeram, S. M., &
Seward, J. F., 2016. Global varicella vaccine
effectiveness: a meta-analysis. Pediatrics, 137(3),
e20153741.
Miller, H. C., & Stephan, M., 1993. Hemorrhagic varicella:
a case report and review of the complications of
varicella in children. The American journal of
emergency medicine, 11(6) ,pp. 633-638.
Nahass, G. T., Goldstein, B. A., Zhu, W. Y., Serfling, U.,
Penneys, N. S., & Leonardi, C. L., 1992. Comparison
of Tzanck smear, viral culture, and DNA diagnostic
methods in detection of herpes simplex and varicella-
zoster infection. Jama, 268(18), pp. 2541-2544.
Nelson, J. D., Bradley, J.S., Barnett, E.D., Cantey, J.B.,
Kimberlin, D.W., Palumbo, P.E., Sauberan, J., et al.,
2016. Nelson’s Pediatric Antimicrobial Therapy. 22
nd
ed. Elk Groove Village: AAP.
Schmader, K.E., & Oxman, M.N., 2012. Varicella and
Herpes Zoster. In: Goldsmith LA, Katz SI, Gilchrest
BA, Paller AS, Leffel JD, Wolff K, editor. Fitzpatrick’s
dermatology in general medicine. 8
th
Edition. New
York: McGraw-Hill, pp. 2383-2401
Seward, J. F., Watson, B. M., Peterson, C. L., Mascola, L.,
Pelosi, J. W., Zhang, J. X., Maupin, J.T., Goldman
S,G., Tabony J.L., Brodovich G.K., Jumaan, A. O., &
Melinda, W., 2002. Varicella disease after introduction
of varicella vaccine in the United States, 1995-
2000. Jama, 287(5), pp. 606-611.
Varicella-Zoster Infections., 2009. in: Report of the
Committee on Infectious Diseases. American Academy
of Pediatrics (Red Book), pp. 714.
Hemorrhagic Varicella in Osteosarcoma: Case Report Is It Induced by Chemotherapy Agent or Nature of the Disease?
423
