
Hand-foot Syndrome Due to Capecitabine: Report of Two Cases
Marsha Bianti, Aninda Marina, Wresti Indriatmi, Sandra Widaty
Department of Dermatology and Venereology, Faculty of Medicine Universitas Indonesia, Dr. Cipto Mangunkusumo
National General Hospital, Jakarta
Keywords: capecitabine, chemotherapy, hand-foot syndrome
Abstract: Capecitabine is one of the most common chemotherapy drugs known to cause hand-foot syndrome (HFS). It
is indicated as adjuvant therapy for the treatment of colorectal cancer, first-line therapy in metastatic colorectal
cancer, and as monotherapy or in combination with docetaxel in metastatic breast cancer. Although considered
to be safe, it has some adverse effect, such as HFS, which is a localized skin eruption associated with the
initiation of therapy with certain chemotherapeutic agents. It is considered common and mild thus frequently
but improperly self-managed and impaired quality of life. A fifty seven years old woman on treatment with
capecitabine for breast cancer and a 46-years-old woman on treatment with capecitabine for breast and thyroid
cancer presented with dry and fissured skin on both palms and soles. They also noted pain and discomfort
while doing their daily activities. On examination, there were multiple erythematous-hyperpigmented plaques,
with scales and fissures overlying it on both palms and soles. Both patients were diagnosed with hand-foot
syndrome due to capecitabine and responded well to topical emollients and steroid
1 INTRODUCTION
Hand-foot syndrome (HFS), also known as
palmoplantar erythrodysesthesia (PPE) or acral
erythema, is a localized skin eruption associated with
the initiation of therapy with certain
chemotherapeutic agents. It is considered a subtype
of toxic erythema of chemotherapy. The clinical
presentation of HFS is characterized by a prodrome
of dysesthesia followed by the development of
painful, symmetrical edema and erythema of the
palms, digits, and soles that may evolve into blisters
and erosions (Hoesly et al., 2011). Although HFS is
considered to affect quality of life, it is not a life-
threatening condition and rarely need hospitalization.
The incidence of HFS depends on the drug therapy,
dosage, and the manner in which the drug is
administered (Farr & Safwat, 2011).
The mechanism of HFS remains unclear. Based
on experience, HFS is considered to be dose-
dependent and probably related to drug metabolite
accumulation in the skin (Farr & Safwat, 2011).
Discontinuation of the offending agent generally
leads to skin regeneration over 1-2 weeks
(Abushullaih, 2002).
Some risk factors were identified to develop HFS,
including the type, dose and duration of
chemotherapy being used and this risk increases with
each dose of potential HFS inducing chemotherapy.
Other risk factors include advanced age, female,
performance status, and exposure to total body
irradiation (Farr & Safwat, 2011; Lassere & Hoff,
2004).
Capecitabine is one of the most common
chemotherapy drugs known to cause HFS. Other
drugs are 5-fluorouracil and liposomal doxorubicin.
Capecitabine is a fluoropyrimidine with
antineoplastic activity. It is a systemic prodrug of 5-
fluorouracil, which has advantage of being orally
administered and has better safety profile (Gressett,
2006). Currently, it is indicated as adjuvant therapy
for the treatment of colorectal cancer, first-line
therapy in metastatic colorectal cancer, and as
monotherapy or in combination with docetaxel in
metastatic breast cancer. Although it is considered to
be safe, capecitabine has side effects, such as nausea,
vomiting, diarrhea, stomatitis, and most commonly,
hand-foot syndrome. This condition is considered
mild thus frequently self-managed, instead of being
referred to Dermatologists. However, the
management is somewhat not proper and the
condition can be worsening and further impaired the
patient’s quality of life. This function impairment is
the main criteria in HFS severity classification. We
report two cases of hand-foot syndrome due to
Bianti, M., Marina, A., Indriatmi, W. and Widaty, S.
Hand-foot Syndrome Due to Capecitabine: Report of Two Cases.
DOI: 10.5220/0008158704150419
In Proceedings of the 23rd Regional Conference of Dermatology (RCD 2018), pages 415-419
ISBN: 978-989-758-494-7
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
415

capecitabine managed with topical emollients and
steroid.
2 CASE
First patient is a 57-years-old woman who was
consulted by Surgery Outpatient Clinic with right
breast cancer TxNxM1 and dry skin on July 12
th
2017. She came to our clinic with chief complaints of
dry and fissured skin, accompanied with pain on both
palms and soles since 1 year ago and got worsen
since. Capecitabine was taken daily since 1 year prior
to admission and 8 months ago she had mild redness,
dry, and flaky skin on both palms. However, it did not
impair her activities.
One month ago, she developed peeling of skin and
itch over both palms and soles. She overcame it by
rubbing skin with baby soap bar and warm water but
the condition didn’t improve. Two weeks ago, the
skin peeling worsen and accompanied by pain
everytime she walks or holds things.
History of contact with irritants, such as
detergents and dishwasher soap, was admitted but she
denied the usage of new brands or products. No
history of atopic and allergy were recorded on patient,
as well as on patient’s family. She bathes twice a day
with bar soap and regular temperature water. Patient
is planned to receive capecitabine for 6 more months.
On September 2012, patient was diagnosed with
right breast tumor suspect of malignancy and referred
to Cipto Mangunkusumo Hospital and then
underwent modified radical mastectomy of the right
breast. She started 25 radiotherapy sessions on July
2013 and 6 cycles of adjuvant chemotherapy on
November 2013. During that time, her laboratory
result showed bicytopenia (anemia and leukopenia)
with normal liver function, and normal renal function
(on August 2014, her estimated glomerular filtration
rate was 84.4 mL/min/1.73m
2
).
In 2015, multiple metastatic nodules on both lungs
were detected through chest radiography and multiple
slice CT scan (MSCT). Therefore, another 6 cycles of
second line chemotherapy (paclitaxel/cisplatin) were
initiated. On June 2016, blastic lesion on right
inferomedial caput femur were found and
capecitabine were started on July 2016. On follow-up
MSCT done on March 2017, multiple nodules on both
thyroid lobes were found, beside metastatic nodules
on both lungs and multiple mediastinal
lymphadenopathy. Treatment with capecitabine was
continued until present time.
During 2017, several abnormalities were found in
her laboratory result. She was pancytopenic and her
renal function was impaired. Her last laboratory test
on June 20
th
2017 showed hemoglobin 9.8 g/dL,
hematocrit 28.1%, leukocyte count 4.81 x 10
3
/μL,
platelet count 119.000/μL, and the estimated
glomerular filtration rate (eGFR) dropped to 42
mL/min/1.73m
2
.
From the physical examination, we found the
patient was fully conscious with normal vital signs.
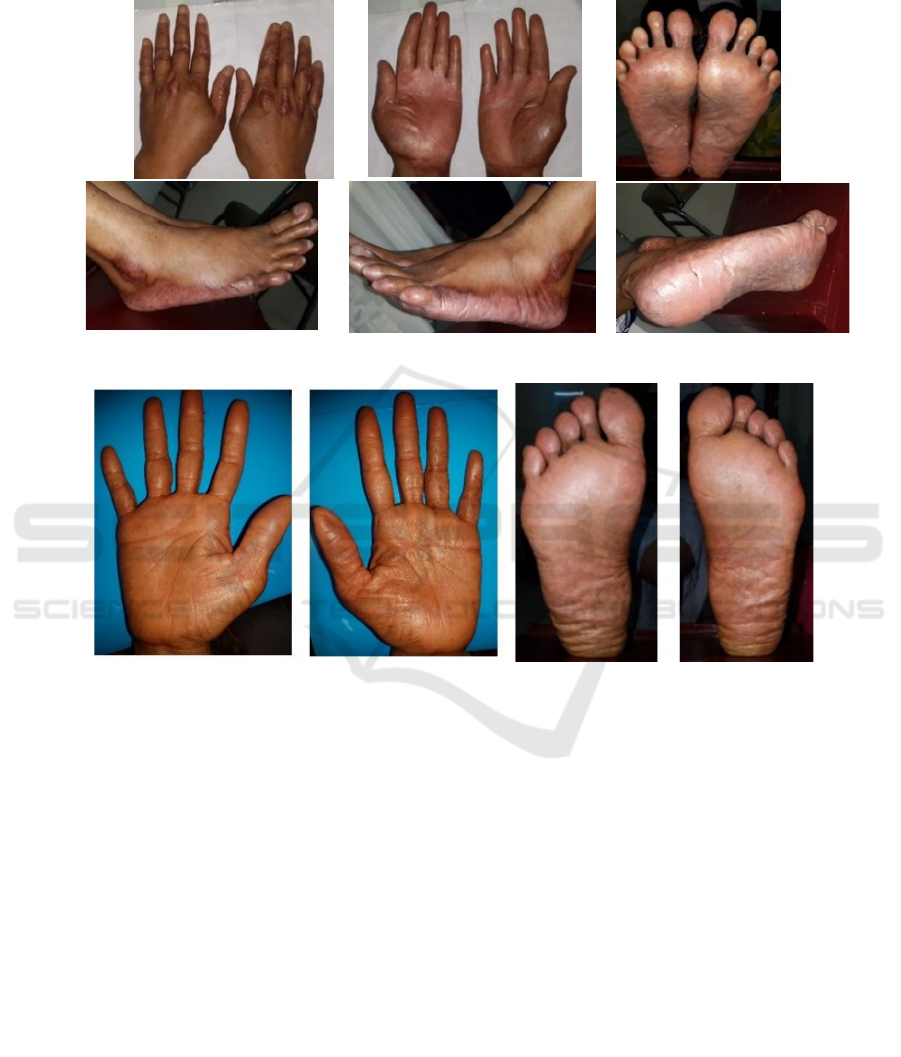
On dermatological examination, we found multiple,
skin colored-erythematous-hyperpigmented plaques,
irregular in shape, plaque in size, circumscribed-
diffused border, with scales and fissures overlying it
on both palms and soles as well as lateral aspect of
the feet. On both back of her hand, on proximal
interphalangeal and metacarpal joints, and bilateral
lateral malleolus, we found multiple, erythematous-
hyperpigmented plaques, lenticular until nummular in
size, circumscribed, discrete, with lichenification,
scales, and fissured overlying it.
Patient was diagnosed with hand-foot syndrome
due to capecitabine and was treated with vaseline
album as emollient and clobetasole propionate 0.05%
ointment twice a day on palms and soles.
Our second patient was a 46-years-old woman
who was consulted by Hematology and Oncology
Division of Internal Medicine Department on
December 7
th
2017. She was consulted with breast
cancer, thyroid cancer, with metastasis to lungs,
brain, mediastinum, and respiratory tract, with chief
complaint of dry skin on palms and soles. She was
complaining of dry and fissured palms and soles since
2 months ago. It is accompanied with itch and pain
while walking.
She had history of prior treatment with
capecitabine from May 2009 to August 2012 but
experienced nothing unpleasant. Capecitabine
treatment was stopped and re-initiated on April 2016
due to respiratory tract metastasis. Two months prior
to admission, she complained redness on both palms
and soles, accompanied with itch and pain on fissured
skin. History of contact with irritants were denied.
She uses vinyl gloves everytime she wash clothes or
dishes. She overcome the complaints by applying
moisturizers and low potency topical corticosteroid
but no improvement were noted. She bathes twice a
day with baby bar soap and regular temperature
water. Patient is planned to receive capecitabine for 5
more years.
From the physical examination, we found the
patient was fully conscious with normal vital signs.
On dermatological examination, we found multiple,
erythematous-hyperpigmented plaques, irregular in
shape, plaque in size, circumscribed-diffused border,
with scales and fissures overlying it on both palms
and soles. Her laboratory test on October 23rd 2017
showed hemoglobin 13.7 g/dL, hematocrit 40.4%,
leukocyte count 4.04 x 103/μL, platelet count
303.000/μL. Her renal function were normal with the
eGFR 88.7 mL/min/1.73m2 and on December 11th
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
416
2017 her eGFR increased to 104.2 mL/min/1.73m2.
Patient was diagnosed with hand-foot syndrome due
to capecitabine and was treated with vaseline album
as emollient and mometasone furoate 0.01% ointment
once a day on palms and soles.
Figure 1. First patient with erythematous-hyperpigmented plaques with scales and fissures on both palms, soles, and lateral
aspect of the feet
Figure 2. Second patient with erythematous-hyperpigmented plaques on both palms and soles
3 DISCUSSION
Various cytotoxic drugs have been reported to have
correlation with HFS. 5-fluorouracil (5-FU) has been
known to cause HFS since the first description in
1984 (Lokich & Moore, 1984). Occurrence of HFS
have been shown to be related to dose and prolonged
drug exposure during continuous intravenous
infusion, or daily ingestion as in capecitabine which
taken by our patients. Our patients were planned to
receive long-term maintenance treatment with
capecitabine, up to 5 years in our second patient.
Gresset reported that the median time of onset is 79
days, ranging from 11 to 360 days (Gressett et al.,
2006). In our first patient, the onset is around 1 year
prior to admission and in our second patient,
HFSoccurred on her second initiation of capecitabine
treatment after 3 years of previous cycle, so we can
conclude that the onset range of HFS is wide.
The mechanism of HFS remains unclear.
However, several theories have been postulated. First,
keratinocytes might have upgraded levels of the
enzyme thymidine phosphorylase which could lead to
capecitabine metabolite accumulation, causing
increased possibility of developing HFS. Second,
capecitabine may be eliminated by the eccrine glands,
which is numerous in palms and soles. Other theory
postulates HFS resulting from increased
vascularization and increased pressure and
temperature in the hands and feet.
From a meta-analysis reported in 1998, HFS
induced by 5-FU seems to be more common in elderly
female patients (Levy et al., 1998). Interestingly,
although our patients suit the gender profile and the
fact that capecitabine is a prodrug of 5-FU, the
relationship between age or gender and HFS seen
with 5-FU has not been clearly seen with
capecitabine.
Hand-foot Syndrome Due to Capecitabine: Report of Two Cases
417

The manifestations of HFS are classified
according to their severity by National Cancer
Institute (NCI) and World Health Organization
(WHO) as shown by Table 1.
Table 1. HFS Grading as defined by NCI and WHO (Webster et al., 2017)
Both of our patients experienced pain and
discomfort in holding objects and upon walking
besides skin changes, however no swelling or
periungual involvement in both patients, so we
classified their HFS as grade 2-3 according to WHO.
According to NCI, both patients classified as grade 3.
The treatment was tailored based on the clinical
manifestations on each patient. The histopathological
findings are nonspecific, therefore it was not
performed.
HFS is usually self-limiting and rarely leads to
life-threatening manifestations. Still, it interferes
treatment schedule and patient’s quality of life. Thus,
proper management is needed to prevent, as well as,
to treat HFS. Currently, the mainstay of the
management of HFS is interruption of therapy and
dose reduction, if necessary. Avoiding potential
irritants, including exposure to extreme changes in
temperature, ill-fitting shoes, tight-fitting clothing,
excessive exercise, and the use of topical anesthetic-
containing cream may prevent the development of
HFS.
Treatment is focused on supportive therapy to
reduce pain and discomfort, also to prevent
infections. Simple topical care with wet dressings,
topical steroids, and emollients are all that required to
clear the condition in some patients. Emollients and
creams have been used for prophylactic and
symptomatic treatment at first signs of grade 1 HFS.
Although the use of steroids for capecitabine-induced
HFS is not proven, steroids may acutely reduce
inflammation. Moreover, several studies reported that
topical or systemic corticosteroids have been useful
for prophylaxis and treatment of HFS for a wide
variety of different drugs. In our patients, the signs of
inflammation were visible, with thickening of the
skin. Therefore we gave high to very high potency
topical steroids, aiming to reduce the inflammation
and relieve symptoms.
In our patients, vaseline album were given. It acts
as occlusive agent that coat the stratum corneum to
retard transepidermal water loss, as well as provide an
emollient effects. In 2-weeks follow up, patients
report improvement in symptoms and skin lesions.
Pain were reduced and their daily activities are no
longer disrupted.
4 CONCLUSION
We report 2 cases of HFS due to capecitabine. Both
patients were diagnosed based on the history and
physical examination and were given topical
emollient and steroid. Both patients responded well
and reported improvement on skin lesions and quality
of life after 2-weeks treatment.
REFERENCES
Abushullaih, S., Saad, E. D., Munsell, M., & Hoff, P. M.,
2002. Incidence and severity of hand–foot syndrome in
colorectal cancer patients treated with capecitabine: a
single-institution experience. Cancer
investigation, 20(1), pp. 3-10.
Farr, K. P., & Safwat, A., 2011. Palmar-plantar
erythrodysesthesia associated with chemotherapy and
its treatment. Case reports in oncology, 4(1), pp. 229-
235.
Gressett, S. M., Stanford, B. L., & Hardwicke, F., 2006.
Management of hand-foot syndrome induced by
capecitabine. Journal of Oncology Pharmacy
Practice, 12(3), pp. 131-141.
Hoesly, F. J., Baker, S. G., Gunawardane, N. D., & Cotliar,
J. A., 2011. Capecitabine-induced hand-foot syndrome
NCI grade NCI definition
1 Skin chan
g
es or dermatitis without pain e.
g
. er
y
thema, peelin
g
2 Skin chan
g
es with pain, not interferin
g
with function
3 Skin chan
g
es with pain interferin
g
with function
WHO grade WHO definition
1 D
y
sesthesia/paresthesia, tin
g
lin
g
in the hands and fee
t
2 Discomfort in holdin
g
ob
j
ects and upon walkin
g
, painless swellin
g
and er
y
thema
3
Painful erythema and swelling of palms and soles, periungual erythema and
swellin
g
4 Desquamation, ulceration, blisterin
g
, severe pain
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
418

complicated by pseudomonal superinfection resulting
in bacterial sepsis and death: case report and review of
the literature. Archives of dermatology, 147(12), pp.
1418-1423.
Lassere, Y., & Hoff, P., 2004. Management of hand-foot
syndrome in patients treated with capecitabine
(Xeloda®). European Journal of Oncology Nursing, 8,
pp. 31-40.
Levy, E., Piedbois, P., Buyse, M., Pignon, J. P., Rougier,
P., Ryan, L., Hansen, R., Zee, B ., Weinerman,
B, Pater, J., & Leichman, C., 1998. Toxicity of
fluorouracil in patients with advanced colorectal
cancer: effect of administration schedule and
prognostic factors.The Meta-Analysis Group in Cancer.
s. Journal of Clinical Oncology, 16, pp. 537-3541.
Lokich, J. J., & Moore, C., 1984. Chemotherapy-associated
palmar-plantar erythrodysesthesia syndrome. Annals of
internal medicine, 101(6), pp. 798-800.
Webster-Gandy, J. D., How, C., & Harrold, K., 2007.
Palmar–plantar erythrodysesthesia (PPE): a literature
review with commentary on experience in a cancer
centre. European Journal of Oncology Nursing, 11(3),
pp. 238-246.
Hand-foot Syndrome Due to Capecitabine: Report of Two Cases
419
