
Nanocrystalline Silver as a Single Treatment
for Decubitus Ulcer: A Case Report
Vidya H. D. Ayuningtyas
1
, Suci Prawitasari
1
, Aunur Rofiq
1
1
Department of Dermatology and Venereology, Faculty of Medicine, Universitas Brawijaya / dr. Saiful Anwar Regional
General Hospital, Malang, Indonesia
Keywords: Nanocrystalline Silver, Decubitus Ulcer, PUSH Scale.
Abstract: The prevalence of decubitus ulcer is very high, especially in inpatient ward and intensive care unit. Adequate
treatment for the wound and prevention measures for decubitus ulcer complication are all mandatory to reduce
the health burden and cost. Previous studies already showed the positive effects of nanocrystalline silver (nAg)
in ulcer healing progress. The purpose of this study is to evaluate the effect of using nAg dressing towards
decubitus ulcer severity. A 31 years old female diagnosed with grade III decubitus ulcer sized 4.7 x 2.7 cm2
was treated with debridement and nAg dressing which regularly changed every week for 4 weeks. Clinical
improvement was evaluated using photography images and Pressure Ulcer Scale for Healing (PUSH) tool.
The evaluation results showed that there was 57% reduction in overall ulcer size in just 4 weeks treatment.
The value of PUSH scale was also found lower down from 14 at the beginning into 10 at the end of the study.
This report highlights the antibacterial effect of nAg dressing as the most potential benefitting factor of the
decubitus ulcer healing. No side effect found during the application of nAg dressing in this patient.
1 INTRODUCTION
Decubitus ulcer or widely known as pressure ulcer,
bedsore, and pressure injury is a localized injury of
the skin and underneath structures due to a
combination of pressure and shear force.1 The
prevalence of decubitus ulcer, range widely from
0.4% at the lowest in acute ward up to almost 24% in
chronic ward. In concordance, the high prevalence of
decubitus ulcer is always followed by the increase of
health cost and burden, which strongly correlated
with the incidence of complications and prolonged
hospital sta .(Norman, 2016).
The failure of healing process as a result of
ischemia, prolonged pressure and trauma is the major
pathogenesis of decubitus ulcer. (Powers,2012)
Bacteria plays a huge role in delaying the wound
healing and thus need to be eradicated by using
multiple kinds of antiseptic or antibiotic. However,
both antiseptic and antibiotic have a flaw as a therapy;
antiseptic may be toxic for the cells and antibiotic has
been known to cause resistance when used
improperly (Dabiri,2016).
Silver, in its pure-active form, has been known to
have antiseptic, antimicrobial, anti-inflammation and
broad-spectrum antibiotic effect without inducing
toxic reaction (Fong, 2006). Though very beneficial,
pure silver is very easy to deactivate thus need
repeated application to achieve the treatment dose
(Argirova,2011). Nanocrystalline technology in nAg-
impregnated dressing provide wider contact area with
the active components while also give better
absorbance of silver ion on the woundbed thus
increasing its efficacy. We reported a 31 years old
woman diagnose with grade III decubitus ulcer
treated with nAg dressing.
2 CASE
A 31 years old female consulted to dermatology
and venereology department of dr. Saiful Anwar
Regional General Hospital by cardiovascular
department for wound on the buttock. The wound has
been recognized since 5 weeks before consulted.
Patient never knew the size and appearance of the
wound but felt mild pain and itch on the wound area
(VAS for the pain is 3). There was no tingling
sensation on the surrounding area of the wound.
History of fever was denied. Patient was once
admitted to dr. Saiful Anwar Regional General
Hospital for 12 days for post-partum cardiomyopathy
Ayuningtyas, V., Prawitasari, S. and Rofiq, A.
Nanocrystalline Silver as a Single Treatment for Decubitus Ulcer: A Case Report.
DOI: 10.5220/0008158003850388
In Proceedings of the 23rd Regional Conference of Dermatology (RCD 2018), pages 385-388
ISBN: 978-989-758-494-7
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
385
about 6 weeks before consulted. During first
admission, the wound was never treated and patient
never received education for mobilisation. After
discharged, patient stayed in bed almost all the time
and only went to the bathroom 2-3 times a day.
Patient stopped wearing diaper since 4 weeks before
consultation and started wearing diaper again two
days before consulted. The diaper was changed 1-2
times a day. History of fecal/urine inconsistency was
denied. Patient never treat the wound and only
consume medication for her heart disease which
consist of furosemide 20 mg once a day, captopril 3 x
25 mg, bisoprolol 5 mg once a day, diazepam 2 mg
once a day, laxadin once a day, NAC 3 x 200 mg, and
cefixime 2 x 100 mg.
General physical examination was normal.
Dermatological examination revealed single
erythematous-based ulcer with irregular border
localized on sacral region. The ulcer sized 5 x 3.5 cm,
covered with yellowish crust and necrotic tissue with
minimal clear exudate. The surrounding skin was
hyperpigmented and no edema. Gram examination
revealed polymorphonuclear cells and gram-negative
coccus. Laboratory examination showed renal
azothemia with ureum level 74,5 mg/dL and
creatinine level 1,01 mg/dL, hyperuricemia 11,0
mg/dl, increase of procalcitonin level 30,53 ng/ml,
hyponatremia 129 mmol/L and hypoalbuminemia
3,04 g/dl. Blood count and blood sugar level was
within normal limit.
Patient was diagnosed with grade III decubitus
ulcer and treated with nAg dressing for 4 weeks in
outpatient ward dermatology and venereology
department of dr. Saiful Anwar Regional General
Hospital. Clinical improvement was evaluated using
photography images and PUSH scale. The evaluation
results showed reduced of ulcer size of about 7.25
cm2 (57% reduction) by the end of the evaluation on
the fourth week (Figure 1). PUSH scale was also
found lower down from 14 at the beginning into 10 at
the end of the study (Table 1).
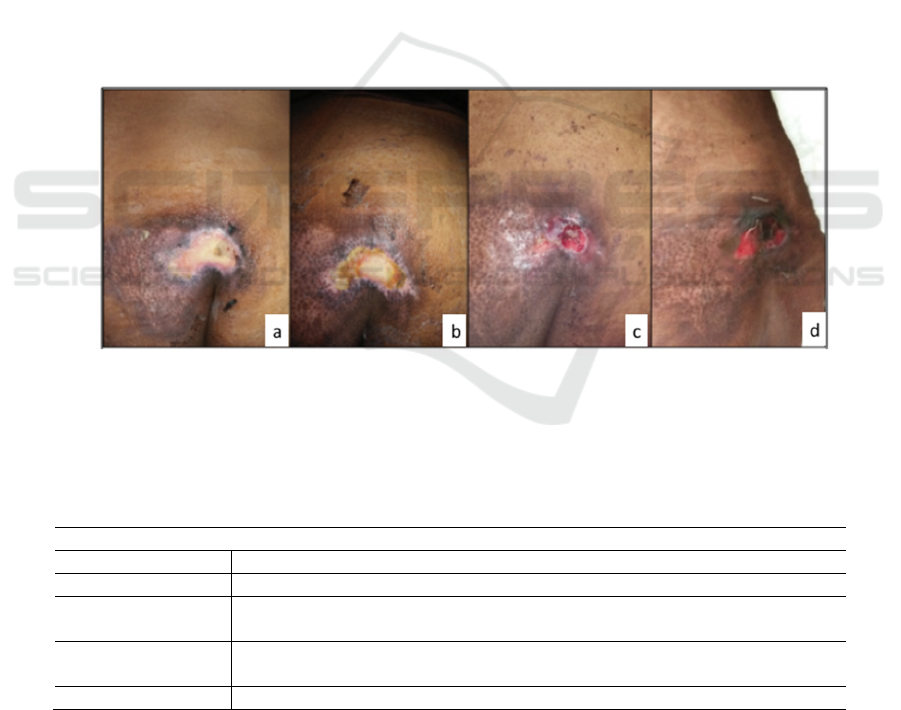
Figure 1. The weekly improvement of decubitus ulcer. The ulcer length and width were reduced significantly in each week
of evaluation, counting for about 35% reduction in the first week, from 12.69 cm
2
(4.7 x 2.7 cm) (a) into 8.2 cm
2
(4.1 x 2.0
cm) (b). The reduction continued as the lesion shrinked into 4.48 cm
2
(3.2 x 1.4 cm) on the third week (c). Though by the
fourth week the ulcer was sli htly enlarged, measured 5.44 cm
2
(3.4 x 1.6 cm) (d), this result did not change the grade of the
ulcer severity. Overall reduction within four weeks was about 57%.
Table 1. Pressure Ulcer Scale for Healing Record
Pressure Ulcer Healing Record
Week 0 1 3 4
Length x Width 12.69 cm
2
(9) 8.2 cm
2
(8) 4.48 cm
2
(7) 5.44 cm
2
(7)
Exudate Moderate (2) Moderate (2) Minimal (1) Minimal
(1)
Tissue Type Slough (3) Slough (3) Granulation
(2)
Granulation
(2)
TOTAL 14 13 10 10
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
386

Table 2: The results of Schirmer test.
Week II III IV
Schirmer test Different > 50% Different > 50% Different <25%
3 DISCUSSION
The National Pressure Ulcer Advisory Panel
(NPUAP) has classified decubitus ulcer into four
grades. First grade decubitus ulcer defined as solely a
non-blanchable erythematous lesion located on bone-
prominence areas. Second grade decubitus ulcer
identified as loss of some part of dermis layer thus the
lesion appears as a shallow, pink-based ulcer, without
slough. Decubitus ulcer categorized into grade three
when the damage affect all layer of the skin thus
exposing subcutaneous fat, but not bone and/or
muscle. Decubitus ulcer grade four defined as an
ulcer accompanied by an exposure of bone and
muscle while also having slough, necrotic tissue and
scar (EPUAP, 2009). This patient was diagnosed with
decubitus ulcer grade three.
The major factor of the pathogenesis of decubitus
ulcer is prolonged inflammation. Within the
environment of chronic ulcer there is an imbalance of
biochemical and molecular components which
mostly caused by bacterial colonization and infection.
Bacterial infection will induce an increase of matrix
metalloproteinase and inflammatory cytokines,
decrease of matrix metalloproteinase inhibitor level
and growth factors. All of the aforementioned
mechanisms lead to delayed healing process and
chronic ulcer development (Fong, 2006.)
The ideal topical therapy needs to fulfil several
criteria such as having antibacterial activity, low
resistance level, low evaporation level, prevent
dehydration, low side effect, can control the pain,
easy to use and having low toxic risk. Silver has a
nature as an antiseptic, antimicrobial, anti-
inflammation and a broad-spectrum antibiotic agent.
The active form of silver, such as Ag+ and Ag0, has
many potent antimicrobial effects that can destroy
microbes through cellular respiration blockade
mechanism and disturb the function of bacterial cell
membrane. The free silver kations bind the protein of
the tissue, altering the structure of bacterial cell
membrane and cause cell death. The silver kations
also able to cause DNA and RNA denaturation thus
hindering cell replication (Fong, 2006) .
The benefit of using wound dressing comprised of
optimal control of moisture, temperature, fluid
permeability, and pH of the wound while also
minimalize the infection, prevent the wound from
excessive trauma and reduce the pain.7 In terms of
silver ion, dressing helps to release the ion gradually,
compensating the nature of silver ion that easily
bound to sodium.
The structure of nAg dressing consists of three
layers with silver-embedded mesh enclosed by two
layers made from rayon/polyester. The
nanocrystalline technology is a modern technology
providing reactive small silver particles thus able to
cover larger area of the wound. The silver kations are
released subsequently and continuously, relieving the
bad odour and exudate, reducing the risk of bacterial
colonization and preventing the wound from
secondary infection (Fong, 2006).
Several studies, either in vivo, in vitro and clinical
test in human support the benefits of using nAg
dressing as a new regiment for chronic wound
treatment. Compared to the other types of topical
silver, e.g. nitrate silver, silver sulfadiazine and
mafenide acetate, nAg has an ability not only to kill
bacteria faster but also effective for broader spectrum
of bacteria, than the others (Wright, 1998;
Thomas,2003).
In vivo study showed that nAg dressing
effectively improve granulation and reduce the
biomolecular inflammatory components10. Clinical
test in human is still very scarce and have low quality
of study. However, several researchers proved that
nAg dressing is correlated with lower pain scale,
reduced volume of the exudate, reduced infection of
the wound and lessen the health cost (Fong, 2006;
Tredget, 1998;Voight,2001). Even now, there is yet
in vivo study that explain the toxicity of nAg towards
keratinocytes and fibroblasts. Nevertheless, in vitro
study has proven that the toxicity level of nAg is very
low. (Fraser,2004)No incidence of resistance ever
reported (Fong, 2006).
Patient was given nanocrystalline dressing
treatment as patient was diagnosed with grade three
decubitus ulcer with high prone to sepsis, indicating
the urgent need of the patient of an adequate and
effective antibacterial agent to prevent new focal
infection development and other severe
complications. Other than dressing, patient and
paramedics also received education of reposition
technique, maximum degree of bed elevation and the
importance of changing the diaper regularly.
During follow up, we found a significant
improvement, showed as decrease of PUSH scale
from 13 at the beginning to 10 at the end of the study.
Nanocrystalline Silver as a Single Treatment for Decubitus Ulcer: A Case Report
387

The ulcer size also found reduced from 12.69 cm2
into 5.44 cm2. These results consentient with
previous studies mentioning the role of topical silver
in re-epithelialization, skin granulation, and
vascularization acceleration thus improving the ulcer
healing. (Demling, 2002; Wright, 2002) No side
effect ever reported during this study.
4 CONCLUSIONS
A 31 years old woman was diagnosed with decubitus
ulcer grade 3 based on history taking and physical
examination. Patient received treatment with nAg
dressing for four weeks. The wound was evaluated
once a week. From the weekly evaluation we found a
significant reduction of ulcer length and width. The
PUSH scale also found 3 points decrease by the end
of the evaluation.
REFERENCES
Argirova, M., & Hadjiiski, O., 2011. Application of the
nanocrystalline silver in treatment of burn wounds in
children. In Skin Grafts-Indications, Applications and
Current Research. InTech.
Dabiri, G., Damstetter, E., & Phillips, T.,2016. Choosing a
wound dressing based on common wound
characteristics. Advances in wound care, 5(1), pp. 32-
41.
Demling, R. H., & DeSanti, M. L., 2002. The rate of re-
epithelialization across meshed skin grafts is increased
with exposure to silver. Burns, 28(3), pp. 264-266.
EPUAP N, NPUAP N., 2009. Prevention and treatment of
pressure ulcers: quick reference guide. Washington DC.
Fraser, J. F., Cuttle, L., Kempf, M., & Kimble, R. M., 2004.
Cytotoxicity of topical antimicrobial agents used in
burn wounds in Australasia. ANZ journal of surgery,
74(3),pp. 139-142.
Fong, J., & Wood, F., 2006. Nanocrystalline silver
dressings in wound management: a review.
international Journal of Nanomedicine, 1(4), pp. 441.
Norman, G., Dumville, J. C., Moore, Z. E., Tanner, J.,
Christie, J., & Goto, S., 2016. Antibiotics and
antiseptics for pressure ulcers. The Cochrane Library.
Powers, J.G., Odo, L., Phillips, T.J., 2012. Decubitus
(Pressure) Ulcers in Fitzpatrick's Dermatology in
General Medicine Eight Edition. Goldsmith. L, Katz. S,
Gilchrest. B, Paller. A, Leffel. D, Wolff. K, editors.
New York: McGraw Hill.
Thomas, S., & McCubbin, P., 2003. An in vitro analysis of
the antimicrobial properties of 10 silver-containing
dressings. Journal of wound care, 12(8), pp. 305-308.
Tredget, E. E., Shankowsky, H. A., Groeneveld, A., &
Burrell, R., 1998. A matched-pair, randomized study
evaluating the efficacy and safety of Acticoat silver-
coated dressing for the treatment of burn wounds.
Journal of Burn Care & Rehabilitation, 19(6), pp. 531-
537.
Ülkür, E., Oncul, O., Karagoz, H., Yeniz, E., & Çeliköz, B.,
2005. Comparison of silver-coated dressing
(Acticoat™), chlorhexidine acetate 0.5%(Bactigrass®),
and fusidic acid 2%(Fucidin®) for topical antibacterial
effect in methicillin-resistant Staphylococci-
contaminated, full-skin thickness rat burn wounds.
Burns, 31(7), pp.874-877.
Voigt, D. W., & Paul, C. N., 2001. The use of Acticoat as
silver impregnated telfa dressings in a regional burn and
wound care center: the clinicians view. WOUNDS-A
COMPENDIUM OF CLINICAL RESEARCH AND
PRACTICE, 13(2), pp. 11-20.
Westby, M. J., Dumville, J. C., Soares, M. O., Stubbs, N.,
& Norman, G., 2017. Dressings and topical agents for
treating pressure ulcers. The Cochrane Library,
6:Cd011947.
Wright, J. B., Lam, K., & Burrell, R. E., 1998. Wound
management in an era of increasing bacterial antibiotic
resistance: a role for topical silver treatment. American
journal of infection control, 26(6), pp. 572-577.
Wright, J. B., Lam, K., Buret, A. G., Olson, M. E., &
Burrell, R. E., 2002. Early healing events in a porcine
model of contaminated wounds: effects of
nanocrystalline silver on matrix metalloproteinases,
cell apoptosis, and healing. Wound Repair and
Regeneration, 10(3), pp. 141-151.
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
388
