
Ophtalmic Herpes Zoster in Patient with Systemic Lupus
Erythematosus
Tania Jessica, Padmawati I. G. A. Dian Intan, Puspawati Ni Made Dwi
Department of Dermatology and Venereology, Medical Faculty of Udayana University/Sanglah Public Hospital, Bali,
Indonesia
Keywords: Ophtalmic herpes zoster, OHZ
Abstract: Ophtalmic herpes zoster (OHZ) might manifest as pain and cutaneous rash limited to periocular region, but
occular involvement have been reported in imunocompromised patients. We present a case report of
ophthalmic herpes zoster in a patient with systemic lupus erythematosus who receive immunosuppressive
therapies to add up to the literature about risk factors and management of OHZ. The diagnosis was
established by the findings from history, physical examination, and supporting examination. The
management of this was conducted collaboratively with Departments of Internal Medicine and
Opthtalmology. The prognosis of this case was dubious due to the higher risk of recurrency associated with
the ongoing immunosuppression therapy.
1 INTRODUCTION
Ophtalmic herpes zoster (OHZ)occurs in 10% to
20% of all herpes zoster cases. This condition might
manifest as pain and cutaneous rash limited to
periocular region, but 50%-72% cases have
demonstrated occular involvement with varied
clinical manifestation and degree of severity (Vrcek
et al, 2017). Several studies have reported the higher
rate of herpes zoster infection among
immunocompromised patients than the general
population, including patients with systemic lupus
erythematosus (SLE) who receive
immunosuppressive therapies (Cohen, 2013).
This
paper reports a case of ophthalmic herpes zoster in a
patient with SLE who underwent chemotherapy with
intraveonous cyclophosphamide to add up to the
literature about risk factors and management of
OHZ.
2 CASE
The patient was a 21-year-old Balinese Indonesian
female with SLE. She was consulted from the
Department of Internal Medicine with vesicles in the
right forehead and around patient’s right eye since
three days before consultation day. These painful
and non itchy vesicles initially appeared in the
forehead. They had been increasing in numbers,
some had coalesced, and furtherthese vesicles
extended to the patient’s right eye. The patient then
complained about red, watery, painful right eye and
trouble with opening it. These complains also
presented with fever. No medications had been
taken, including topical treatement. The patient had
a history of varicella during childhood.
The patient was currently receiving 500mg pulse
dose metilprednisolone within 250cc sodium
chloride 0.9% for 3 days, followed by intravenous
62.5mg metilprednisolone every 12 hours for 5 days,
then intravenous 62.5 mg metilprednisolone every
24 hours for, anda maintenance dose of 16 oral
prednisolones every 12hours. She had also received
one cycle of chemotherapy with 500 mg
cyclophosphamide within 250cc sodium chloride
0.9%.
During the examination, the patient demonstrated
normal vital sign, mild pain (visual analog
scale/VAS: 2), and right eye visual acuity of 6/15.
Vesicles and crusts presented in the edematous right
eyelid. The dermatological examination of the right
frontal region, right upperlid (in concordance with
the dermatome of the ophthalmic branch of the
trigeminal nerve) revealed efforescence of multiple
vesicles, some had coalesced and formed rounded
geographical bullaes with sizes ranging from 0.1 x
Jessica, T., Dian Intan, P. and Dwi, P.
Ophtalmic Herpes Zoster in Patient with Systemic Lupus Erythematosus.
DOI: 10.5220/0008157803770380
In Proceedings of the 23rd Regional Conference of Dermatology (RCD 2018), pages 377-380
ISBN: 978-989-758-494-7
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
377
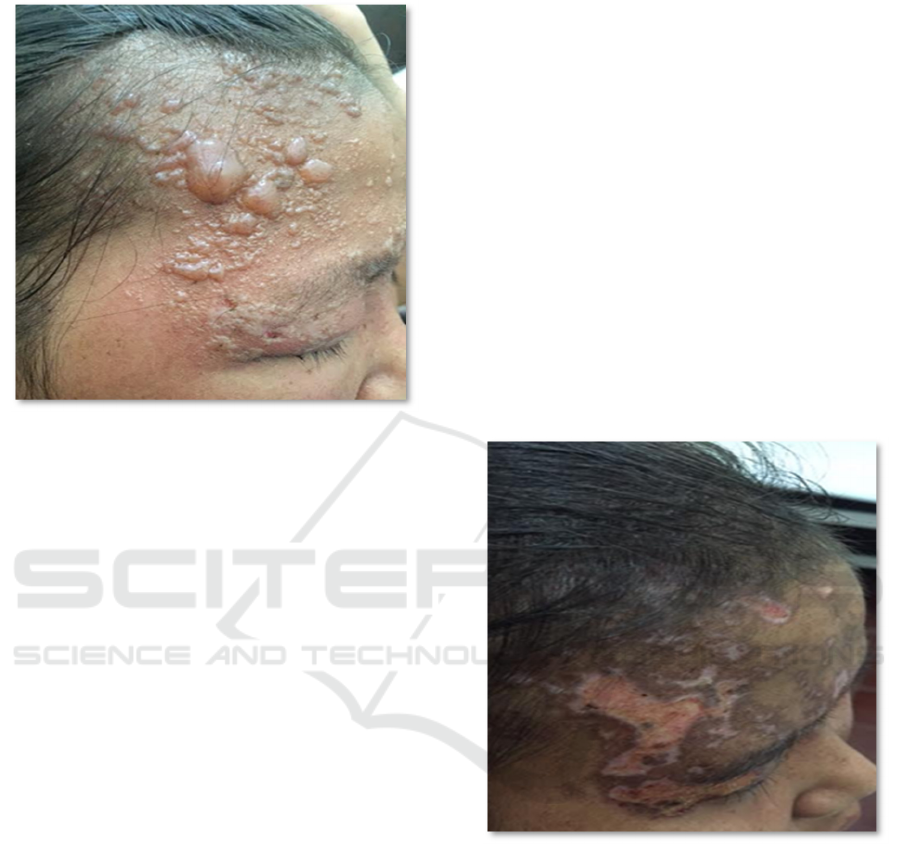
0.2 cm to 0.5 x 0.8 cmabove the erythematous skin
surface, as illustrated in Figure 1.
Figure 1: Findings of dermatological state examination
day 1.
Complete blood count revealed lowered values
red blood cellsmeasure-ments and blood chemistry
demonstrated increasedvalues of liver enzyme tests,
blood urea nitrogen, and lowered albumin. The
Tzanck smear of specimen from scrapping of the
bullae base showed multinucleated giant cells.
The diagnosis of the patient was ophthalmic
herpes zoster that was in concordance with the
dermatome of trigeminal nerve’s ophthalmic branch.
She was treated with 800 mg oral acyclovir every 5
hours (day 1) for 10days, 500 mg oral paracetamol
tablet every 8 hours (if fever arose), oral vitamin B1,
B6, B12 tabletsevery 24 hours, 500 mg oral
mefenamic acid tablet every 8 hours (if required),1%
salicyl powder + 0.5% menthol applied in the
bullous lesions every 12 hours. The patient also
received explanations about her condition, planned
treatment, and possible complications. She was also
consulted to the Department of Ophtalmology who
subsequently diagnosed her with right ophthalmic
blepharoconjunctivitis and provided gentamycin eye
ointment every 8 hours and 1 drop of levofloxacin
and lyteers every 4 hours for the right eye. The
maintenance prednisolone dose was continued by
Department of Internal Medicine, and the second
cycle of cyclophosphamide chemotherapy was
delayed until the skin lesions had improved.
Further follow up on day 11 revealed that the
bullae had resolved and left erosions on the
erythematous skin surface partially covered by dark
brown crusts. No new lesions nor fever were
reported, and the pain improved.
Acyclovir and paracetamol were no longer
administered but the mefenamic acid and vitamin
tablets were resumed. Fifteen minutes open dressing
with 0.9% Sodium cchloride 0,9% was conducted
every 8 hours on the erosive lesions, and 2% Sodium
Fusidate cream was applied to the lesion every 12
hours afterwards. The patient also continued to
receive levofloxacin and lyteers drops.
The examination on day 18 revealed that much
of the lesions had dried (Figure 2). No pain, no
itchiness, no new lesions, and no fever were
encountered. The mefenamic acid was no longer
administered while vitamin tablets, open dressing
with 0.9% sodium chloride, and the application of
2% Sodium Fusidate cream was continued. The
Department of Ophtalmology stopped the
administration of levofloxacin drop, but continued
the lyteers and added gentamycin eye ointment
every 8 hours.
Figure 2: Findings of dermatological state examination
day 18.
3 DISCUSSION
Cutaneous manifestation of SLE presents in 75%
patients and serves as an early sign in about quarter
of the cases (Kuhn et al, 2013).
These patients have
11-23 higher risk for infection than the general
population, and herpes zoster is the most common
viral infection encountered (Sayeeda et al, 2010).
Herpes zoster infection might present in the form of
ophthalmic herpes zoster (OHZ) marked by the
occurrence of inflammation in the eye, intraneural,
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
378

and perineural of the sensory nerve. OHZ frequently
presents with dermal eruptions that are in
concordance with the dermatome, but occular
involvement is uncommon.
An imminulogical study in patient with SLE
showed a breakdown of cell mediated immune,
delayed of hypersensitivity reaction, and hyperactive
humoral immune system. The side effect from high
dose corticosteroids therapy and other
immunosuppresive agents alsocan decrease host
resistency to some infections. The activity of
disesase, nephritis lupus, and positive Sm-antibody
have been reported as risk factors of herpes zoster in
SLE (
Leroux, 2016).
This case reported a 21-year-old female with
SLE who was consulted from the Department of
Internal Medicine due to the emergence of vesicles
in the right forehead and around the patient’s right
eye. This patient was also under treatment oh high
dose and long term therapy with immunosuppresive
agents such as cyclophosphamide and
corticosteroids.
The diagnosis of OHZ was then established
from the history, physical examination, and
supporting examination. This patient presented with
effloresence of multiple vesicles, some of them had
coalesced and formed bullaes on the erythematous
skin of right frontal region and right upper lid (in
concordance with the dermatome of the ophthalmic
branch of the trigeminal nerve). These clinical
features were in concordance with the diagnosis of
OHZ (Vrcek et al, 2017). In addition, the Tzanck
smear with Giemsa staining revealed multinucleated
giant cells. Other modalities to establish the
diagnosis might include the histopathological
examination, viral culture, polymerase Chain
Reaction (PCR), and serological tests (
Schmader and
Oxman, 2012
).
However, due to the possibly longer
duration to obtain results and cost effectiveness
consideration, these test were not conducted in the
patient.
The management of OHZ is similar to the herpes
zoster infection in general, but additional eye
management should be conducted. The management
should attempt to decrease viral replications,
accelerate recovery, relieve pain, and prevent
complications (Dail and Makes, 2002). This includes
the main therapy with antivirals, added with
supporting therapies such as analgetics and topical
therapies both for the skin and the eye (Dworkin et
al, 2007). The patient in the case received 800 mg
acyclovir ever 5 hours for 10 days, added with oral
mefenamic and vitamins B1, B6, B12.Open dressing
and sodium fusidate cream were provided for the
skin treatment, while gentamycin eye ointment and
lyteeers were provided for the eye.
The prognosis of OHZ is generally favorable, but
patients older than 70 years old or who are
immunocompromised are at higher risk of
recurrence (Armando et al, 2015). The most
common complication of herpes zoster infection is
post herpetic neuralgia. In 9% cases, this pain might
last for a period that ranges from 4 weeks to 10
years. In this case report, the patient was a 21-year-
old female who showed improvement after
collaborative treatments. However, due to the
ongoing immunosuppression therapy for her SLE,
her prognosis was dubious, with a higher risk of
recurrent herpes zoster infection than the general
population.
4 CONCLUSION
This case report presented the occurrence of
opthtalmic herpes zoster in a patient with SLE. The
diagnosis was established by the findings from
history, physical examination, and supporting
examination. The management of this was
conducted collaboratively according to the available
recommendations. The prognosis of this case was
dubious due to the higher risk of recurrency
associated with the ongoing immunosuppression
therapy.
REFERENCES
Armando, S., Nicoletta, V., Sara, P., Matilde, G., Silvia,
L., Giovani, G., 2015. Herpes zoster: New Preventive
Perspective. Journal of Dermatology and Clinical
Research, 3(1), pp.1024-1046.
Cohen, J. I., 2013. Herpes Zoster. The New England
Journal of Medicine, 369(3), pp. 255–63. doi:
10.1016/j.mcna.2013.02.002.
Daili, S.F., Makes, W.I., 2002. Herpes Zoster Pada Pasien
Imunokompeten dalam Infeksi Virus Herpes. Penerbit
Fakultas Kedokteran Universitas Indonesia, pp. 190-
199.
Dworkin, R.H., Johnson, R.W., Breuer, J., Gnann, J.W.,
Levin, M.J., Backonja, M., Betts, R.F., Gershon, A.A.,
Haanpaa, M.L., McKendrick, M.W., Nurmikko, T.J.,
Oaklander, A.L., Oxman, M.N., Pavan-Langston, D.,
Petersen, K.L., Rowbotham, M.C., Schmader, K.E.,
Stacey, B.R., Tyring, S.K., van Wijck, A.J.M.,
Wallace, M.S., Wassilew, S.W., Whitley, R.J., 2007.
Recommendations for the management of herpes
zoster. Clinical infectious diseases: an official
publication of the Infectious Diseases Society of
America 44 Suppl 1, S1-26. doi:10.1086/510206
Ophtalmic Herpes Zoster in Patient with Systemic Lupus Erythematosus
379

Kuhn, A., Bonsmann, G., Anders, H.J., Herzer, P.,
Tenbrock, K., Schneider M., 2015. The Diagnosis and
Treatment of Systemic Lupus Erythematosus. Dtsch
Arztebl Int, 112, pp. 423-32.
Leroux, M.B., 2016. Herpes zoster in patients with
systemic lupus erythematosus. Our Dermatol Online
4.
Sayeeda, A., Al Arfaj, H., Khalil, N., Al Arfaj, A.S., 2010.
Herpes Zoster Infections in SLE in a University
Hospital in Saudi Arabia: Risk Factors and Outcomes.
Autoimmune Diseases 2011, 174891.
doi:https://dx.doi.org/10.4061/2010/174891
Schmader, K.E., Oxman, M.N.,. Varicella and Herpes
Zoster. In: Goldsmith LA, Katz SI, Gilchrest BA,
Paller AS, Leffell DJ, editors., 2012. Fitzpatrick’s
Dermatology in General Medicine. Mc Graw-Hills.
New York, 8
th
ed., pp.2383-2401.
Vrcek, I., Choudhury, E. and Durairaj, V., 2017. Herpes
Zoster Ophthalmicus: A Review for the Internist.
American Journal of Medicine, pp. 21–26. doi:
10.1016/j.amjmed.2016.08.039.
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
380
