
Changing Pattern of Candida Species in Vulvovaginal Candidiasis
using Vitek 2
Linda Astari, Dewi Puspitorini, Cita Rosita Sigit Prakoeswa, Sunarso Suyoso
Dermatovenereology Departement Airlangga University/ Dr. Soetomo Hospital Surabaya, Indonesia
Keywords: Candida sp, vulvovaginal candidiasis, Vitek 2
Abstract: Vulvovaginal candidiasis (VVC) is a common fungal infection. VVC causes inflammation of vulva and
vagina, characterized with erythematous vulva and vagina, with symptoms of pruritus and vaginal discharge.
Candida albicans remains the cause of VVC in almost 80% cases. Candida non-albicans now are emerging
threat as a cause of VVC, due to extensive usage of antifungal drugs. The importance of identifying Candida
species within clinical samples is in order to provide information concerning the proper treatment for patients.
Rapid identification of Candida species are essential in clinical laboratories. Vitek 2 is an automated
microbiology identification system that evaluates an optical signal generated by biochemical reactions
contained within a variety of identification cards. Vitek 2 could be used to identify Candida sp to the species
level. This microbiological system gives some advantages, it is faster than other diagnostic tools for VVC, it
works automatically and accurately, and it practically do not need manual work so it also minimizes error.
But for teaching hospital, it still need conventional method to study fungal morphology and to do Vitek 2
examination (from cornmeal-Tween 80 agar or CHROMagar Candida).
1 INTRODUCTION
Candida species are microorganisms which live
normally in our body, the skin, mouth,
gastrointestinal tract, and genitourinary tract,
including vulva and vagina. It usually lives as benign
commensals and produce no diseases. However, in
women with some predisposition factors, such as
vaginal douching usage, pregnancy, and
immunosuppressed condition, the colony of Candida
sp will grow higher and causing VVC. This disease is
usually a common problem on child-bearing age
women and 5% will have recurrent infectious
episodes (Vermitsky J, Self MJ, Chadwich SG,
Trama JP, Adelson ME, Mordechai E, et al, 2008;
Zeng J, Zong LL, Mao T, Huang YX, Xu ZM, 2011).
Candida albicans is responsible for infection in
80 to 90% of VVC cases, but now VVC due to
Candida non-albicans have increased steadily over
the latest decades (Srihartati E, Hoetomo MM,
Ervianti E, 2006; Ervianti E, Sawitri, Murtiastutik D,
Agusni RI., 2011; Jimoh O, Inabo HI, Yakubo SE,
Ankuma SJ, Olayunka AT., 2016; Cassone A, 2016).
The rise in VVC infections that more specifically
caused by Candida non-albicans species, could be
due to an increase in over-the-counter antifungal use.
Nowadays rapid identification of microorganisms
that cause diseases are important in clinical
laboratories. Better and faster diagnostic system for
VVC would let physicians could be able to make
therapeutic decisions based on species causative
agents of VVC so enhance proper treatment for their
patients. Phycisians necessitate the rapid and accurate
identification of yeasts to the species level by the
clinical microbiology laboratory. Vitek 2 is an
automated microbiology identification system that
evaluates an optical signal generated by individual
biochemical reactions contained within a variety of
microbe identification cards. After inoculation with a
standardized suspension of the unknown organism,
each self-contained card is incubated and read by the
instrument’s internal optics. Vitek 2 provides a highly
automated, objective yeast identification method with
excellent performance. This system is useful for
timely and accurate identification of significant yeast
species in the clinical microbiology laboratory. Vitek
2 showed faster and better species identification result
than other diagnostic systems such as conventional
methods and nonculture methods because they can be
time-consuming and manual-labour (Meurman O,
Koskensalo A, Jalava-Rantakoko K, 2006;
Rajkumari N, Mathur P, Xess I, Misra MC., 2014)
328
Astari, L., Puspitorini, D., Prakoeswa, C. and Suyoso, S.
Changing Pattern of Candida Species in Vulvovaginal Candidiasis using Vitek 2.
DOI: 10.5220/0008156803280332
In Proceedings of the 23rd Regional Conference of Dermatology (RCD 2018), pages 328-332
ISBN: 978-989-758-494-7
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

2 METHODS
This study was a cross-sectional descriptive study that
identifying causes of VVC to the species level by
using Vitek 2. Patients attending the Sexually
Transmitted Infection (STI) Division
Dermatovenereology Clinic of Dr Soetomo General
Hospital Surabaya that suspected VVC were
examined by the physician. The samples of this study
were all VVC patients that fulfilled the inclusion
criteria. The inclusion criteria were VVC patient,
women age 15 years or more than 15 years, married
or unmarried and willing to follow the research and
signed the informed consent. The exclusion criteria
were patients with negative culture result.
Patients were examined for VVC signs and
symptoms. Samples were taken from vaginal swab,
then underwent the direct microscopic examination
(wet preparation, and Gram stain). Samples were
tested for conventional methods and Vitek 2.
Conventional methods consisted of Sabouraud
dextrose agar (SDA) then Cornmeal-Tween 80 agar,
carbohydrate fermentation test, and CHROMagar
Candida (CAC). The first method is using SDA then
Cormeal-Tween 80 agar. This method showed
structures of Candida species on microscope in 3
days (72 hours). The other method was carbohydrate
fermentation test that consisted of 6 carbohydrates.
The result of this test positive if there was changing
color of broth to yellow and the tube inside the broth
(Durham tube) filled with gas in 2-3 days (48– 72
hours). The third one was CHROMagar Candida that
showed colonies in 1,5-2 days (36-48 hours). Color
of colony showed the Candida sp. Overall all of this
conventional method need 1,5-3 days (36-72 hours)
to identify the species of Candida.
Colony from Cornmeal-Tween 80 agar or CAC
were also checked by using Vitek 2. Four until six
fresh colonies (16-24 hours age of colonies) and were
taken and suspension with NaCl 0,85% in order to get
standardized suspension (1.8-2 McF) were made.
After inoculation with a standardized suspension of
the unknown organism, each self-contained card is
incubated and read by the instrument’s internal
optics. The reading result were compared to the
baseline data of Vitek 2.
3 RESULTS
The most common age of patients is on young adult
15-24 age group. History of high frequency or
recurrency of VVC is on 72% of patients. Clinical
examination showed edematous and erythematous
vulva and vagina on all patients. There were variation
result on direct microscopic examination from wet
preparation and Gram stain. There were positive and
negative result. There were no negative culture result.
Sabouraud dextrose agar then Cornmeal-Tween
80 agar examination showed specific characteristic of
each species of Candida. Terminal vesicles
(chlamydoconidia) with pseudohifa, blastoconidia
looked like a flower, could be concluded as Candida
albicans. Other samples with divaricated pseudohifa
and oval blastoconidia, could be concluded as
Candida tropicalis. Few samples with budding yeast
like cell and no pseudohypha may reveal the species
of Candida glabrata. The carbohydrate fermentation
test showed positive result based on the changing
color to yellow on 6 carbohydrates tested and the gas
filled in the Durham tube. Positive result on dextrose
and trehalose means that the sample grown was
Candida glabrata. Positive result on dextrose,
maltose, galactose and trehalose means that the
sample was Candida albicans. Other positive result
showed on dextrose, sucrose, maltose, galactose and
trehalose means that the sample was Candida
tropicalis, and one positive result showed on dextrose
means Candida parapsilosis or could be other
Candida sp, so it will need other conventional
method, to get the final result. The last positive result
on dextrose and sucrose only, this means could be
other Candida sp, so that it will need other
conventional method to support the definite result.
CHROMagar Candida revealed the growth of
Candida sp by its colony color. All samples revealed
1 type of colony color or 1 Candida sp in every
sample, but only 4 samples showed 2 colony color (2
Candida sp). Colony color light green means the
fungi was Candida albicans (if dark green means C
dubliniensis). Purple colony color means the fungi
was Candida glabrata. White colony color means
Candida parapsilosis. Blue color means Candida
tropicalis. This method should be completed with 2
other conventional methods (SDA then Cornmeal
Tween 80 agar and carbohydrate test). The
conventional methods from 25 sample showed 14
samples of Candida albicans, and others were
Candida non-albicans. Five samples were Candida
glabrata, 1 sample was Candida parapsilosis and 4
samples were grown with 2 Candida sp or infected by
2 Candida sp. Result of samples: 1 sample were
combination Candida albicans with Candida
glabrata, 1 sample were combination of Candida
albicans with
Candida famata, and 2 samples: each
sample contained Candida albicans with Candida
tropicalis.
Changing Pattern of Candida Species in Vulvovaginal Candidiasis using Vitek 2
329
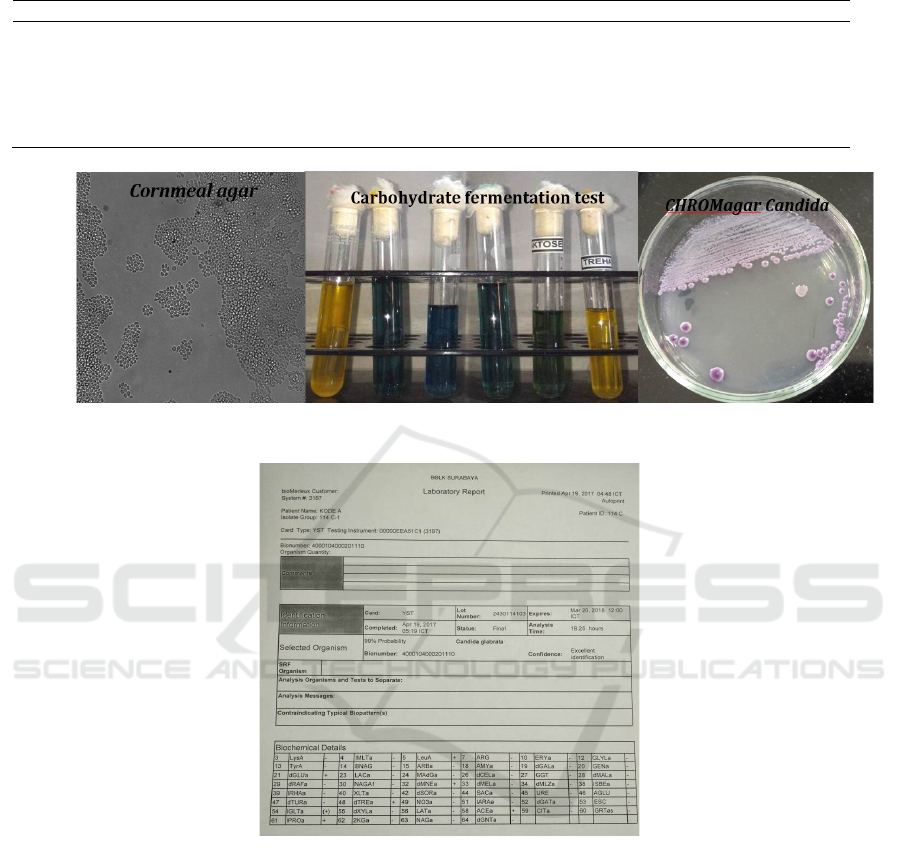
Table 1: Comparation between Conventional methods and Vitek 2.
Conventional Methods Vitek 2
All samples revealed growth of Candida sp to
the species level: C albicans (the highest), C
glabrata, C tropicalis, C parapsilosis, C famata
Time-consuming (36-72 hours)
Laborious
All samples revealed growth of Candida sp to
the species level: C albicans (the highest), C
glabrata, C tropicalis, C parapsilosis, C
famata
Faster (18 hours)
Automatic machine, minimizing error
Figure 1: Conventional methods (SDA then Cornmeal agar, Carbohydrate fermentation test, CHROMagar Candida).
Figure 2: Result sheet of Vitek 2.
In Vitek 2, 25 of samples showed result of Candida
sp 100% same as conventional methods. Fourteen
samples were Candida albicans, and others were
Candida non-albicans. Five samples were Candida
glabrata, 1 sample was Candida parapsilosis and 4
samples were grown with 2 Candida sp or infected by
2 Candida sp. Result of 4 samples: 1 sample were
combination Candida albicans with Candida
glabrata, 1 sample were combination of Candida
albicans with Candida famata, and 2 samples: each
sample contained Candida albicans with Candida
tropicalis. From table 1 showed that the result of
species from 25 samples of Vitek 2 was 100% the
same as in conventional methods. Vitek 2 has some
advantages that it is faster and works automatically.
4 DISCUSSION
The most common age of patients was an age of
young adult 15-24 group from total of 25 patients. It
could be explained that the 15-24 age group is child-
bearing age, which is high of estrogen hormone,
allowing increase growth of Candida sp. History of
high frequency or recurrency of VVC was on 72% of
subjects. It means that a lot of women with VVC were
often not going to the doctor seeking for treatment,
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
330

because maybe they think this disease is not life-
threatening and would heal itself, and it could be
because of the predisposition factors that still exist on
them (Khairnar R, Khairnar A.,
2017; Fidel PL,
Cutright J. Steele C., 2000; Kalia N, Singh J, Sharma
S, Kamboj SS, Arora H, Kaur M., 2015). Clinical
examination showed edematous and erythematous
vulva and vagina on all patients. This is concordance
that clinical signs of VVC are edematous and
erythematous vulva and vagina. Direct microscopic
result from wet preparation and Gram stain showed
positive and also negative result, but there were no
negative colony culture result. This showed that
direct microscopic examination is only an additional
tool supporting the examination. The negative result
of direct microscopic examination did not exclude the
diagnosis of VVC, but signs of clinical examination
establish the diagnosis (Kundu RV, Garg A., 2013;
Sobel JD, 2008).
There are 3 conventional methods that support
each other. SDA then Cornmeal Tween 80 agar
showed specific characteristic of each species of
Candida. The carbohydrate fermentation test showed
positive result based on the changing color to yellow
on 6 carbohydrate tested and the gas filled in the
Durham tube. CAC revealed the growth of Candida
sp by its colony color. The conventional methods
from 25 samples showed 14 samples of Candida
albicans, and others were Candida non-albicans, 5
samples were Candida glabrata. One sample were
Candida parapsilosis and 4 samples were grown with
2 Candida sp or infected by 2 Candida sp. Result of
samples: 1 sample were combination Candida
albicans with Candida glabrata, 1 sample were
combination of Candida albicans with Candida
famata, and 2 samples: each sample contained
Candida albicans with Candida tropicalis (Suyoso S.
Mucosal candidiasis. In: Bramono K, Suyoso S,
Indriatmi W, Ramali LM, Widaty S, Ervianti E,
editors., 2013; Larone DH, 2011)
In Vitek 2 from 25 samples showed result of
species identification Candida sp 100%, the same as
conventional methods. The highest number species
were on 14 samples (Candida albicans). The
conventional methods need about 36-72 hours to
identify the species , but Vitek 2 only needs 18 hours.
Vitek 2 has more advantages than conventional
methods. Vitek 2 immediately yielded the result of
species by the machine itself, Vitek 2 has faster time
than conventional methods to identify to the species
level and can work automatically so it does not need
manual labour, and it minimizes error (Mona et al.,
2015; Esmat MM, Mohamed T, Abdelrahman AH,
2005).
5 CONCLUSION
The best method of identification of the species is
using the combination of this 3 conventional
methods. Conventional identification methods are
still considered to be the reference standard for the
identification of yeast isolates and also for education
purposes, but are laborious and time-consuming.
Beside that, conventional methods can show
structures of Candida sp clearly that this structures
could not be seen using Vitek 2. Conventional
methods are important and useful for learning fungi
especially in teaching hospital, Dr Soetomo Surabaya
Hospital. A fast and accurate technique for yeast
identification is very important for microbiological
laboratories. According to the results found in the
present study, the Vitek 2 system (from cornmeal-
Tween 80 agar or CAC) identified most clinically
important Candida sp reliably within 18 hours, and
appears to be an excellent alternative identification
method for performing fungal diagnostics.
The result showed the most common yeast
causing VVC was Candida albicans (56%), but there
were increasing of Candida non-albicans that cause
VVC. Most Candida non-albicans are usually
causing antifungal resistance. It is therefore important
that there should be increased awareness among
physicians on the rising prevalence of Candida non-
albicans, due to the reduced susceptibility to azoles.
Prior identification to the species level on VVC is
essential to ensure early diagnosis of Candida non-
albicans infection and in order to give proper
treatment.
REFERENCES
Cassone, A., 2015. Vulvovaginal C andida albicans
infections: pathogenesis, immunity and vaccine
prospects. BJOG: An International Journal of
Obstetrics & Gynaecology, 122(6), pp. 785-794.
Ervianti E, Sawitri, Murtiastutik D, Agusni RI, 2011. The
shifting pattern of Candida sp. causing vulvovaginal
candidiasis and recurrent vulvovaginal candidiasis.
Berkala Ilmu Kesehatan Kulit dan Kelamin, 23(3),
189-193.
Esmat, M. M., Mohamed, T., & Abdelrahman, A. H., 2015.
Species Identification and Antifungal Susceptibility
Profile of Candida Isolates from ICU Patients in Sohag
University Hospital, Upper Egypt. The Egyptian
Journal of Medical Microbiology, 24(4), pp. 89-97.
Mona, F., Nasr, E., Ashgan, B., & Mohamed, E. Ahmed H,
2015. Antifungal Susceptibility Pattern and Species
Distribution of Candida Isolates from Patients with
Changing Pattern of Candida Species in Vulvovaginal Candidiasis using Vitek 2
331

Vulvovaginal Candidiasis. International Journal of
Advanced Research, 3, pp. 1376-1386.
Fidel, P. L., Cutright, J., & Steele, C., 2000. Effects of
reproductive hormones on experimental vaginal
candidiasis. Infection and immunity, 68(2), pp. 651-
657.
Jimoh, O., Inabo, H.I., Yakubo, S.E., Ankuma, S.J.,
Olayunka, A.T., 2016. Prevalence and speciation of
non-albican vulvovaginal candidiasis in Zaria. Journal
National Science Research; 6(2), pp. 51-56.
Kalia, N., Singh, J., Sharma, S., Kamboj, S. S., Arora, H.,
& Kaur, M., 2015. Prevalence of vulvovaginal
infections and species specific distribution of
vulvovaginal candidiasis in married women of north
india. Int. J. Curr. Microbiol. App. Sci, 4(8), pp. 253-
266.
Khairnar, R., & Khairnar, A., 2017. Vaginal candidiasis
among pregnant women a prevalence study. Sch J App
Med Sci; 5, (2a), pp. 336-338.
Kundu, R.V., & Garg, A., 2012. Yeast infections:
candidiasis, tinea (pityriasis) versicolor, and
malassezia (pityrosporum) folliculitis. In: Goldsmith
LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff
K, editors. Fitzpatrick dermatology in general
medicine. 8th ed. New York: McGraw Hill; p.2300-1.
Larone, D.H., 2011. Medically important fungi. 5th ed.
New York: ASM Press; p.117-136.
Meurman, O., Koskensalo, A., & Rantakokko‐Jalava, K.,
2006. Evaluation of Vitek 2 for identification of yeasts
in the clinical laboratory. Clinical microbiology and
infection, 12(6), pp. 591-593.
Rajkumari, N., Mathur, P., Xess, I., & Misra, M. C., 2014.
Distribution of different yeasts isolates among trauma
patients and comparison of accuracy in identification of
yeasts by automated method versus conventional
methods for better use in low resource countries. Indian
journal of medical microbiology, 32(4), pp. 391-397.
Sobel, J.D., 2008. Vulvovaginal candidiasis. In: Holmes,
K.K., Sparling, P.F., Stamm, W.E., Piot, P., Wasserheit,
J.N., Corey, L., et al, editors. Sexually transmitted
diseases. 4th ed. New York: McGraw Hill; p.823-838.
Srihartati, E., Hoetomo, M.M., & Ervianti, E., 2006.
Sensitivity test of antifungal to Candida sp. using
microdelution methods in vulvovaginal candidiasis.
Berkala Ilmu Kesehatan Kulit dan Kelamin, 18 (1), pp.
1-12
Suyoso, S., 2013 Mucosal candidiasis. In: Bramono, K.,
Suyoso, S., Indriatmi, W., Ramali, L.M., Widaty, S.,
Ervianti, E., editors. Dermatomikosis superfisialis.
Jakarta: Badan penerbit FKUI; p.120-135.
Vermitsky, J. P., Self, M. J., Chadwick, S. G., Trama, J. P.,
Adelson, M. E., Mordechai, E., & Gygax, S. E., 2008.
Survey of vaginal-flora Candida species isolates from
women of different age groups by use of species-
specific PCR detection. Journal of clinical
Microbiology, 46(4), pp. 1501-1503.
Zeng, J., Zong, L. L., Mao, T., Huang, Y. X., & Xu, Z. M.,
2011. Distribution of Candida albican genotype and
Candida species is associated with the severity of
vulvovagianl candidiasis. Nan fang yi ke da xue xue
bao= Journal of Southern Medical University, 31(10),
pp. 1649-1653.
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
332
