
A New Insight on Atopic Skin Diathesis:
Is It Correlated with the Severity of Melasma
Danar Wicaksono
1*
, Rima Mustafa
2
, Sri Awalia Febriana
1
, Kristiana Etnawati
1
1
Dermatovenereology Department, Faculty of Medicine
Universitas Gadjah Mada – Dr. Sardjito General Hospital, Yogyakarta-Indonesia
2
Clinical Epidemiology and Biostatistics Unit, Faculty of Medicine Universitas Gadjah Mada –Dr. Sardjito General
Hospital, Yogyakarta-Indonesia
Keywords: Melasma, atopic skin diathesis (ASD), MASI score, atopic dermatitis (AD)
Abstract: Melasma is a macular lesion of light brown to dark on the sun-exposed area, especially on the face. Atopic
Skin Diathesis (ASD) is a clinical term to describe skin atopics with previous, present or future atopic
dermatitis (AD). Dennie-Morgan infraorbital folds are secondary creases in the skin below the lower eyelids
with a sensitivity of 78% and a specificity of 76% to diagnose AD. Melasma skin is characterized by
impaired stratum corneum integrity and a delayed barrier recovery rate. Barrier dysfunction will stimulate
keratinocyte to secrete keratinocyte-derived factor, which plays role in skin pigmentation process in
melasma. To analyze correlation between ASD and Melasma Area Severity Index (MASI) score in melasma
patient. This study is an observational analytic study with cross sectional design. Measurement of ASD and
MASI score were done in 60 subjects with melasma who went to dermatology outpatient clinic Dr. Sardjito
General Hospital from July 2017 to Januari 2018. The correlation between ASD and MASI score was
analyzed using Pearson correlation. The result of this study showed no significant correlation between ASD
and MASI scores (r: 0.02, p: 0,85). Crude Relative Risk (RR) for Dennie-Morgan infraorbital folds and
MASI score was 4 (1.01-15.87). There was no correlation between ASD and MASI scores. Patient with
Dennie-Morgan infraorbital folds has 4 times higher risk for developing severe melasma.
1 INTRODUCTION
Melasma is a hyperpigmented disorder of macular or
patches lesions with light brown to dark, irregular
edges and firm borders. Lesions are usually
symmetrical on the sun exposed area especially on
the face, primarily affects female patients and tends
to be chronic and relapsing. In Indonesia the
prevalence of melasma is estimated about 0.2 - 4%
of all cases of skin diseases (Kim et al., 2007;
Hernández-Barrera et al., 2008).
Melasma can cause
pigmentation which is detrimental to patients’
psychological well-being and bring adverse
consequences in their social life, recreational
activities, and emotional well-being for a long
period of time (Kang et al., 2002). Due to the
chronicity of the disease, treatment of melasma
should take a minimum of 8 weeks with a
considerably high cost. Despite the high cost of
treatment, this disease is chronic and recurrent
without any guarantee of full remission. Most
clinician usually use (Melasma Area Severity Index)
MASI score to evaluate treatment in melasma. The
MASI score showed good reliability within and
between raters and was found to be valid when
compared with the melasma severity scale,
mexameter scores, and area measurements
(Berardesca & Maibach,1996).
The cause of melasma is not yet known, but
several factors are thought to play a role in the
etiopathogenesis of melasma.
The interaction of
keratinocytes may also be involved in melasma: the
activation of inducible nitric oxide synthase (iNOS)
and Keratinocyte Derived Factor (KDF) within
keratinocytes particularly after ultraviolet (UV)
radiation, has a role in melanogenesis process (Reed
et al., 1995). Impaired skin barrier is one of the
underlying mechanism in melasma pathogenesis.
This condition is also commonly found in atopic
dermatitis patients. Several scoring systems have
been proposed to assess the degree of skin barrier
impairment. Among the most commonly used
190
Wicaksono, D., Mustafa, R., Febriana, S. and Etnawati, K.
A New Insight on Atopic Skin Diathesis: Is It Correlated with the Severity of Melasma.
DOI: 10.5220/0008153701900194
In Proceedings of the 23rd Regional Conference of Dermatology (RCD 2018), pages 190-194
ISBN: 978-989-758-494-7
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

scoring system is Atopic Skin Diathesis (ASD) score
which are calculated based on atopic symptoms and
signs for practical use and for clinical or
epidemiological studies (Kompaore et al., 1993). A
total of 13 components are assessed in the
calculation of ASD score, many of which are
associated with imparied skin barriers, including
keratosis pilaris, xerotic skin, pityriasis alba, and
Dennie-Morgan infraorbital folds. However, the
scoring system is usually used to diagnose atopic
dermatitis.
Given that skin barrier impairment is found in
both melasma and atopic dermatitis we thought that
ASD score might also be of use in assessing
melasma patients. In this study, we would like to
investigate the correlation betwen the ASD score
and severity of melasma.
2 METHOD
This is an analytic cross-sectional study that
included 60 melasma patients who went to
dermatology outpatient clinic at Dr. Sardjito General
Hospital from July 2017 to Januari 2018.
Measurement of both ASD and MASI score were
done for those patients. Descriptive characteristics
for the subjects were presented. The correlation
between ASD and MASI score was analyzed using
Pearson correlation. Further, MASI score was
divided into two categories, <18 (low) and ≥18
(high). Crude relative risk (RR) was calculated to
assess the risk of higher MASI score associated with
each component of ASD score.
3 RESULT
The descriptive summaries of enrolled subjects were
presented in Table 1. The age of the subjects ranged
from 32 to 61 years old with an average of 46.65
years old. Centrofacial was the most common type
of melasma (83.3%) followed by malar type
(16.67%). Skin type IV accounted for 70% of the
subjects.
Table 1. Descriptive summaries of the subjects
Mean ± SD
Number of subjects (%)
Age, years
46.65 5.96
-
Minimum age 32 -
Maximum age 61 -
Length of disease, years 7.8 -
Minimum duration 0.5 -
Maximum duration 20 -
Skin Type
III - 10(16.67)
IV - 42(70)
V - 8(13.33)
Melasma Type
Mala
r
- 10(16.67)
Mandibula
r
- 0
Centrofacial - 50(83.3)
MASI score
<18 - 53(88.83)
≥18 - 7 (11.67)
ASD score
<10 - 55(91.67)
≥10 - 5(8.33)
ASD: atopic skin diathesis
MASI: melasma area severity index
A relatively large proportion of subjects were found
to have lower scores of MASI (88.83%) and ASD
91.67%,). While the majority of subjects were in the
group of having lower MASI and ASD scores, a
Pearson correlation test that had been carried out did
not result in a significant correlation between ASD
and MASI scores (r:-0.02, p:0.85) (Figure 1).
A New Insight on Atopic Skin Diathesis: Is It Correlated with the Severity of Melasma
191
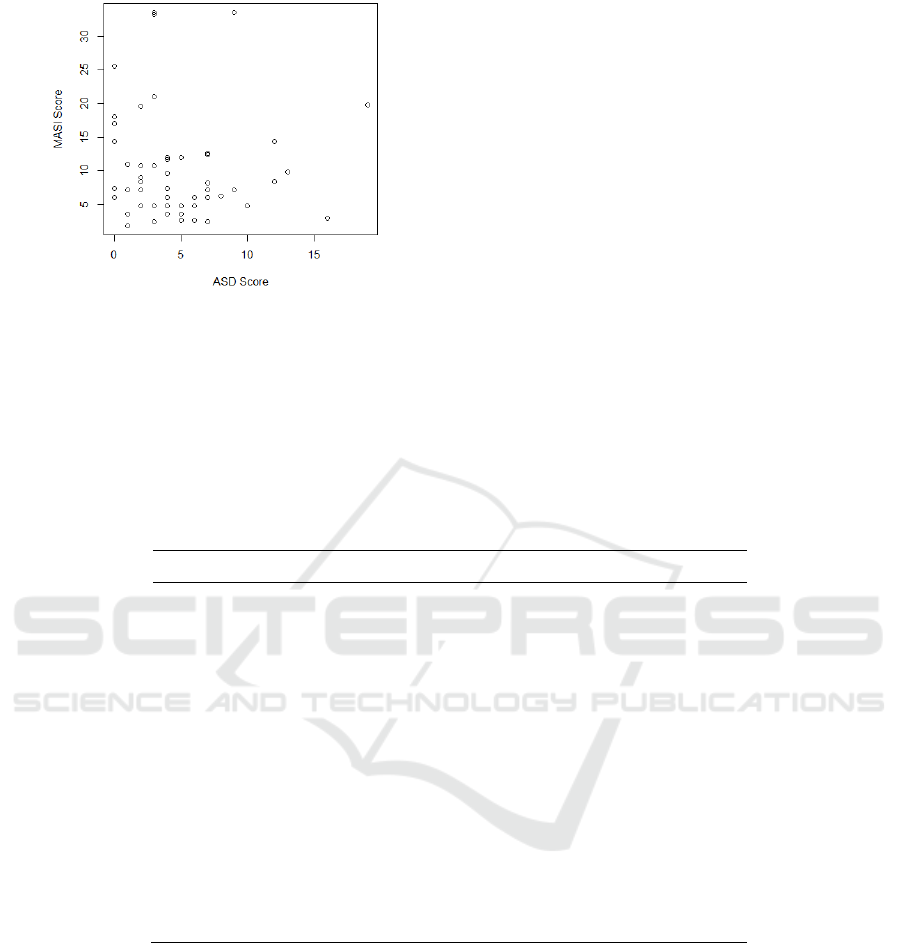
Figure 1. The scatterplot of ASD score vs MASI score.
Additional analysis was also carried out to
analyze the relationship between ASD score and
MASI score after categorizing them into two groups
(lower or higher score groups) as in Table 1.
However, we did not find any significant association
between those two categorical variables (results not
presented here).
This figure did not show any pattern of
correlation between both scores, as later supported
by a Pearson correlation test (r:-0.02, p: 0.85).
Further, we calculated the crude relative risks (RR)
for having more severe melasma, indicated by a
higher category of MASI score (≥18), for each
component that contributed to the calculation of
ASD score. Of all components, the presence of
intraorbital folds was the only factor that appeared to
be significantly associated with higher risk of severe
melasma (RR: 4.00(1.01-15.87)).
Except for keratosis pilaris, other components of
ASD score related with skin barrier impairments (the
presence of xerotic skin and pityriasis alba) showed
a trend towards a higher risk of severe melasma,
even though the relationship did not appear to be
statistically significant (Table 2). In particular, we
could not calculate the RR for keratosis pilaris since
none of our subjects in this study were found with
that condition.
Table 2. Relative risk for having higher MASI score (≥18) for each component of ASD score
ASD Score Components Relative Risk (95% CI)*
Total number of subjects = 6
0
Cradle cap 0.94 (0.13-6.94)
Intraorbital fol
d
4.00 (1.01-15.87)
Perleche 0.39 (0.02-6.29)
Pityriasis alba
#
1.83 (0.27-12.36)
Ear rhagade 2.00 (0.17-24.17)
Palmar hyperlinearit
y
0.94 (0.13-6.94)
White Demographism 3.03 (0.72-12.76)
Xerosis
#
2.00 (0.45-8.89)
Itchy while sweating 0.21 (0.01-3.45)
Fotofobia 0.55 (0.07-4.17)
Wool Intolerance 3.03 (0.72-12.76)
Food Intolerance 0.31 (0.02-5.02)
Allergic Rhinitis 0.29 (0.04-2.24)
Asthma 1.50 (0.22-10.46)
Sensitivity to Metal 0.33 (0.04-2.58)
Atopic History in Famil
y
3.11 (0.77-12.51)
ASD: atopic skin diathesis
*Values are crude (unadjusted) relative risk (95% confidence interval)
#
Components that are related with skin barrier impairment
4 DISCUSSION
Our study contributes to building evidence on the
use of ASD score in evaluating melasma. To our
knowledge, this is the first study that assess the
relationship between ASD score and severity of
melasma. A larger sample size would be required for
a more comprehensive analysis on the use of ASD
score in evaluating melasma. In our study, more than
80% of subjects were found to have both lower
MASI and ASD score. This is one of the major
limitations of our current study. Since the patients
recruited for this study were regular patients at Dr.
Sardjito General Hospital who had received
treatment based on the standard protocol, i.e, they
were not newly diagnosed patients with melasma.
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
192

The mean duration of disease of the subjects were
7.8 years, so it was very likely that the disease has
evolved throughout those years.
The characteristic of the subjects in this study
showed that centrofacial type was the most common
type of melasma (83.3%) followed by malar type
(16.67.%), in accordance with previous research
which reported that most types of melasma were
centrofacial type followed by malar and mandibular
type (Kim et al., 2007; Hernández-Barrera et al.,
2008). Centrofacial type is most often found in
women, whereas malar type is more often found in
men. This is thought to be related to the predominant
occupational activity outside the home in male
patients.
The result of this study is similar to the previous
studies with majority of the subjects aged between
40 to 50. However, as we only included patients who
went to Dr. Sardjito Hospital during our study
period, our sample could not be considered as
representative of the real melasma patients in the
population. Our study, in which the majority of
subjects were found to have skin type IV, is in
accordance with previous studies in Brazil. Our
subjects (70%) have skin type IV and little portion
of skin type III (16.67%) and type V (13.3%) (Reed
et al., 1997; Kang et al., 2010).
Our results suggest Dennie-Morgan infraorbital
folds as the only ASD score component being
significantly associated with higher risk of severe
melasma. However, interpretation of our results
should be done very cautiously. The relative risks
calculated in this study were crude relative risks, i.e.,
the calculation was carried out without taking into
account (adjusting to) any other parameters that
might simultaneously affect the risk of having more
severe melasma. Further analysis with adjustment to
other potential confounders is, therefore, necessary.
Dennie-Morgan infraorbital folds
are secondary
creases in the skin below the lower eyelids. They are
a minor criterion of AD and are present in up to 84%
of patients with AD, with a sensitivity of 78% and a
specificity of 76%. They are also described in
patients with allergic rhinitis and/or asthma without
AD (Kang et al., 2006; Merle et al., 2010). The
pathophysiology is not clearly established. They
may be related to skin edema and the continuous
spasm of the Muller eyelid muscle resulting from
hypoxia linked to poor blood circulation. Finally,
our research supports the idea that impaired skin
barrier might be a common underlying mechanism
that mediates the link between atopic conditions
with melasma. Further investigation is necessary to
provide the evidence on this relationship.
5 CONCLUSION
We did not find any correlation between ASD and
MASI scores. More in-depth research on ASD score
can be used to investigate the alleged causal
relationship in melasma.Examination of ASD score
is not a routine examination of melasma patients and
other oxidative stress disorders, so another indicator
is required in measuring skin barrier function.
REFERENCES
Berardesca, E., Maibach, H., 1996. Racial differences in
skin pathophysiology. Journal of the American
Academy of Dermatology. doi:10.1016/S0190-
9622(96)80070-3
Hernández-Barrera, R., Torres-Alvarez, B., Castanedo-
Cazares, J.P., Oros-Ovalle, C., Moncada, B., 2008.
Solar elastosis and presence of mast cells as key
features in the pathogenesis of melasma. Clinical and
Experimental Dermatology 33, 305–308.
doi:10.1111/j.1365-2230.2008.02724.x
Kang, H.Y., Bahadoran, P., Suzuki, I., Zugaj, D., Khemis,
A., Passeron, T., Andres, P., Ortonne, J.P., 2010. In
vivo reflectance confocal microscopy detects
pigmentary changes in melasma at a cellular level
resolution. Experimental Dermatology 19.
doi:10.1111/j.1600-0625.2009.01057.x
Kang, H.Y., Hwang, J.S., Lee, J.Y., Ahn, J.H., Kim, J.Y.,
Lee, E.S., Kang, W.H., 2006. The dermal stem cell
factor and c-kit are overexpressed in melasma. British
Journal of Dermatology 154, 1094–1099.
doi:10.1111/j.1365-2133.2006.07179.x
Kang, H.Y., Suzuki, I., Lee, D.J., Ha, J., Reiniche, P.,
Aubert, J., Deret, S., Zugaj, D., Voegel, J.J., Ortonne,
J.P., 2011. Transcriptional profiling shows altered
expression of wnt pathway- and lipid metabolism-
related genes as well as melanogenesis-related genes
in melasma. Journal of Investigative Dermatology
131, 1692–1700. doi:10.1038/jid.2011.109
Kang, W.H., Yoon, K.H., Lee, E.S., Kim, J., Lee, K.B.,
Yim, H., Sohn, S., Im, S., 2002. Melasma:
Histopathological characteristics in 56 Korean
patients. British Journal of Dermatology 146, 228–
237. doi:10.1046/j.0007-0963.2001.04556.x
Kim, E.H., Kim, Y.C., Lee, E.S., Kang, H.Y., 2007. The
vascular characteristics of melasma. Journal of
Dermatological Science 46, 111–116.
doi:10.1016/j.jdermsci.2007.01.009
Kompaore, F., Marty, J.P., Dupont, C., 1993. In vivo
evaluation of the stratum corneum barrier function in
blacks, caucasians and asians with two noninvasive
methods. Skin Pharmacology and Physiology 6, 200–
207. doi:10.1159/000211136
Merle, C., Laugel, C., Baillet-Guffroy, A., 2010. Effect of
UVA or UVB irradiation on cutaneous lipids in films
or in solution. Photochemistry and Photobiology 86,
A New Insight on Atopic Skin Diathesis: Is It Correlated with the Severity of Melasma
193

553–562. doi:10.1111/j.1751-1097.2009.00690.x
Reed, J., Ghadially, R., Elias, P., 1997. Integrity and
permeability barrier function of photoaged human
epidermis. Archives of Dermatology 133, 395–396.
doi:10.1001/archderm.1997.03890390139031
Reed, J.T., Ghadially, R., Elias, P.M., 1995. Skin Type,
but Neither Race nor Gender, Influence Epidermal
Permeability Barrier Function. Archives of
Dermatology 131, 1134–1138.
doi:10.1001/archderm.1995.01690220040008
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
194
