
Adverse Cutaneous Drug Reactions Due to Antituberculosis Therapy
in Dr. Sardjito General Hospital Yogyakarta
Kusnindita Noria Rahmawati
1
, Danar Wicaksono
1
, Fajar Waskito
2
, Sri Awalia Febriana
2
1
Resident of Departement of Dermatovenereology, Universitas Gadjah Mada, Yogyakarta, Indonesia
2
Division of Allergy Immunology, Departement of Dermatovenereology, Universitas Gadjah Mada, Yogyakarta, Indonesia
Keywords: Tuberculosis, Antituberculosis, Adverse Cutaneous Drug Reaction, Rifampicin, Cutaneous Reaction.
Abstract: Tuberculosis (TB) is a major health problem worldwide. From 2014-2017, 577 cases of TB were recorded in
Dr. Sardjito Hospital Yogyakarta. Use of specific agents against Mycobacterium tuberculosis is mainstay of
TB treatment. First line anti-TB therapy used are rifampicin, isoniazid, pyrazinamide, and ethambutol. This
regimen can cause various adverse drug reaction affecting several organs, including skin. Presence of adverse
cutaneous drug reactions (ACDRs) to anti-TB therapy can reduce the effectiveness of therapy and increase
the morbidity and mortality of TB patients. This study aimed to understand the type of ACDRs that frequently
occur due to anti-TB therapy, the most common causative drugs, and to describe the clinical characteristics
including the patch test results. This is a retrospective cross-sectional study on TB patients receiving anti-TB
therapy in Dr. Sardjito General Hospital Yogyakarta from 2014-2017. Medical record investigation was
conducted to find cutaneous reactions appeared during the course of anti-TB therapy. There were 33 out of
577 patients recorded with ACDRs, maculopapular rash was the most common type (66.7%), followed with
Stevens Johnson-Syndrome (12%); Drug Reaction Eosinophilia & Systemic Symptoms (DRESS) (6%) and
acneiform eruption (6%); erythroderma(3%), exfoliative dermatitis(3%), and bullous drug eruption (3%).
Fifteen out of 33 patients had underwent patch tests examination. Rifampicin was found to be the most
causative agent, followed by pyrazinamide, ethambutol and isoniazide. As conclusion, maculopapular rash is
the most frequent anti-TB therapy-related ACDRs with rifampicin as the most frequent causative drug based
on patch test results in Dr. Sardjito General Hospital Yogyakarta.
1 INTRODUCTION
Tuberculosis (TB) is still a major health problem in all
over the world, including Indonesia. TB occurs almost
in all countries but more than 80% of TB cases
reported to occur in 22 countries worldwide. In 2013,
56 % of new cases of TB occurred in Southeast Asia
and Western Pacific (WHO, 2004). Ranked at number
3 as the biggest contributor of TB after India and
China, the prevalence of TB in Indonesia was
272/100,000 population in 2013, while the incidence
rate was 183/100,000 population (Departemen
Kesehatan RI, 2016). From 2014-2017, 577 cases of
TB were recorded in Dr. Sardjito General Hospital
Yogyakarta.
According to the National Guidelines of
Tuberculosis Diagnosis and Management, use of
specific agents against Mycobacterium tuberculosis is
the mainstay of TB treatment. For the first line, anti-
TB therapy used are rifampicin (RIF), isoniazid
(INH), pyrazinamide (PZA), and ethambutol (EMB).
To improve the compliance of TB patient, fixed dose
combination (FDC) were produced and available in 2
forms, which consist of 4 drugs (RIF 150 mg, INH 75
mg, PZA 400 mg, EMB 275 mg) and 3 drugs (RIF 150
mg, INH 75 mg, PZA 400 mg) in each tablet
(Departemen Kesehatan RI, 2016). This multidrug
therapy of TB could cause various adverse drug
effects, ranging from the mild to severe condition.
Adverse drug reactions related to anti-TB therapy
could happen in several organs, most common of them
are hepatotoxicity, gastrointestinal intolerance
peripheral neuropathy, optic neuritis and cutaneous
lesions (Ton, 2008).
World Health Organization (WHO) classifies
toxicity adverse drug reactions into 2 subtypes, type A
and type B reactions. Type A reaction is the most
common and related to pharmacological properties of
a drug. It can occur in everyone, usually predictable
and often dose-dependent. Symptoms may improve
186
Rahmawati, K., Wicaksono, D., Waskito, F. and Febriana, S.
Adverse Cutaneous Drug Reactions Due to Antituberculosis Therapy in Dr. Sardjito General Hospital Yogyakarta.
DOI: 10.5220/0008153601860189
In Proceedings of the 23rd Regional Conference of Dermatology (RCD 2018), pages 186-189
ISBN: 978-989-758-494-7
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

with drug dose reduction. Type A reactions may
include drug interactiotoxicity, and side effects. Type
B reaction occurs in 10-15% of patients. It has
unpredictable characteristics, tends to occur in people
who have predisposing factors. Type B reactions, both
non-immunologic and hypersensitivity-based, are
usually not dose-dependent and require drug
discontinuation for resolution. Type B reactions
include drug intolerance, hypersensitivity, or
idiosyncratic reactions to drugs (Kardaun et al., 2013).
Adverse cutaneous drug reactions (ACDRs) of
anti-TB therapy can present as a mild reactions such
as flushing and/or itching of the skin with or without
rashes, also can appear in conjunction with hot
flashes, palpitations, headache and/or increased blood
pressure. This can be considered as type A reactions.
Moderate/severe reactions to anti-TB therapy are
more related to type B reactions. Clinical
presentations vary from hives and rashes until the
severe conditions like exfoliative dermatitis and those
involving mucous membranes, such as Stevens-
Johnson Syndrome (SJS) (Lawrence Flick Memorial
Tuberculosis Clinic, 1993).
The presence of ACDRs to anti-TB therapy could
reduce the effectiveness of therapy; with regard of
patients’ reduced compliance or drugs cessation that
leads to treatment failure or relapses.
Moreover, ACDRs as well could increase the
morbidity and mortality of TB patients. This study
aimed to understand the type of ACDRs that
frequently occur due to anti-TB therapy, the causative
drugs, and to describe the clinical characteristics
including the patch test results to anti-TB therapy. We
hope that it can increase the awareness and assist
medical provider to perform the proper management.
2 METHODS
This is a retrospective cross-sectional study on TB
patients receiving anti-TB therapy in Dr. Sardjito
General Hospital Yogyakarta from 2014-2017. A
study on patient’s medical record was done to find
ACDRs appeared during the course of anti-TB
therapy. ACDRs due to anti-TB therapy was
diagnosed based on anamnesis, physical and
dermatological examination and laboratory
examinations. DRESS was diagnosed based on
RegiSCAR criteria.
3 RESULT
This study included 577 patients receiving anti-TB
therapy in Dr. Sardjito General Hospital, Yogyakarta.
Thirty-three patient were experiencing ACDRs due to
anti-TB therapy.Characteristics of patients with
cutaneous manifestations as an adverse effects of
anti-TB therapy are described in Table 1.
Table 1: Baseline characteristics of patient with ACDRs due to anti-TB therapy.
Variable
Number of patients
n (%)
Sex
Female
11 (33.3%)
Male
22 (66.7%)
Age
≤ 18 years
6 (18.2%)
19-35 years
14 (42.4%)
35-49 years
7 (21.2%)
≥50 years
6 (18.2%)
Types of ACDRs
Maculopapular rash
22 (66.7%)
Erythroderma
1 (3%)
Drug Reaction Eosinophilia & Systemic
Symptoms (DRESS)
2 (6%)
Stevens-Johnson Syndrome (SJS)
4(12%)
Exfoliative dermatitis
1 (3%)
Bullous drug eruption
1 (3%)
Acneiform eruption
2 (6%)
Adverse Cutaneous Drug Reactions Due to Antituberculosis Therapy in Dr. Sardjito General Hospital Yogyakarta
187
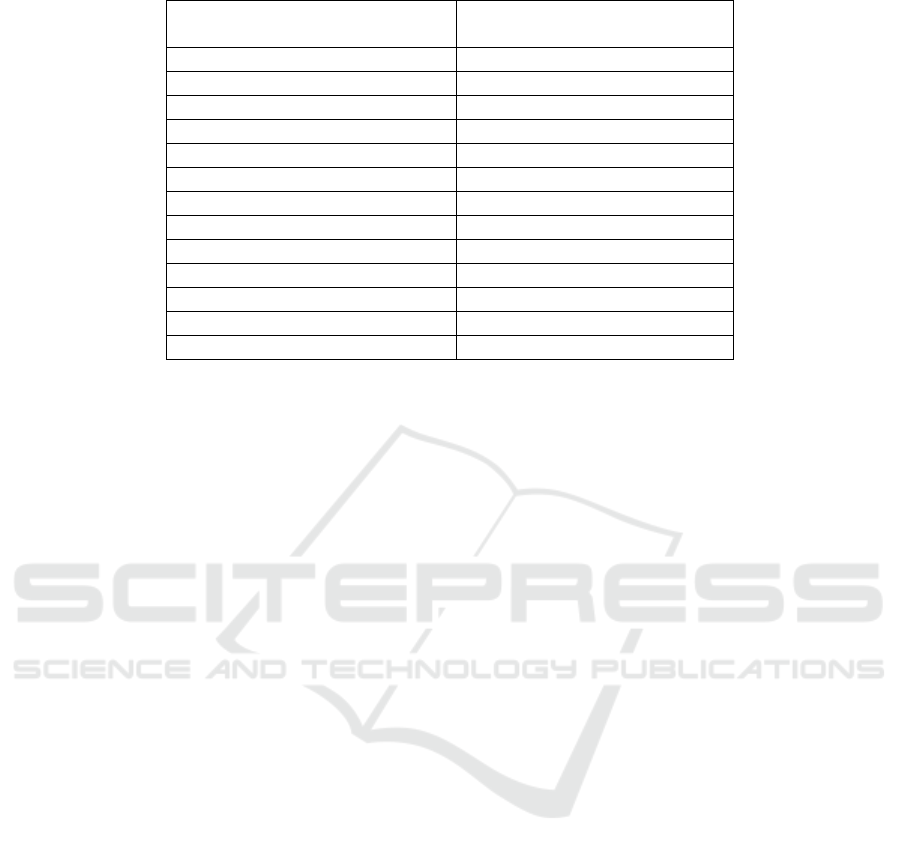
Table 2: Patch test results and culprit anti-TB drug.
Patch Test Results and Culprit
Drug
n (%)
Positive
Rifampicin
3 (20%)
Isoniazid
1 (6%)
Pyrazinamide
1 (6%)
Ethambutol
1 (6%)
4 FDC (RHZE)
*
3 (20%)
Doubtful
Rifampicin
2 (13%)
Isoniazid
-
Pyrazinamide
1 (6%)
Ethambutol
-
4 FDC (RHZE)*
1 (6%)
Negative
5 (27.7%)
Note: each patient can have more than one positive results to anti-TB therapy
*
RHZE : rifampicin, isoniazid, pyrazinamide, ethambutol
The age of patients ranged from 3 to 58 years
(mean = 31.09 years; median = 29 years). Most of the
patients were male (66.7%) and came from 19-35
years age group (42.4%). Maculopapular rash
(66.7%) was the most common type of ACDRs in
patients receiving anti-TB therapy, followed by SJS,
DRESS, and acneiform eruption. Erythroderma,
exfoliative dermatitis, and bullous drug eruption was
found in one patient each. Fifteen out of 33 patients
were underwent patch test examination with anti-TB
therapy to found the causative drugs. Patch test results
are described Table 2.
Based on the patch testing results, rifampicin was
found to be the most anti-TB therapy causing
ACDRs, followed by pyrazinamide. Positive patch
test results to isoniazid and ethambutol only found in
each one patient.
4 DISCUSSION
Side effects and hypersensitivity to anti-TB therapy
that manifests in many organs still remains a
difficulty in treating TB patients, so as those affecting
the skin. In severe ACDRs, it is recommended to
withdraw the suspected drug in order to improve
symptoms and outcomes (Dheda, 2012). Interruption
of TB therapy could carry consequences in worsening
the prognosis of patients, development of drug
resistance, and risk of transmission to others. Tan et
al. found that there is a significant association
between TB treatment interruption and risk of death
during the intensive phase of treatment (p = 0.001)
(Tan et al., 2007). Clinicians should be very careful
in determining the severity of ACDRs and to decide
whether to stop one or all type of anti-TB therapy also
when to re-introduce the therapy with thorough
consideration.
From the result of the study, ACDRs found in 33
patient out of 577 patients receiving anti-TB therapy
(5.7%). There were some other studies that also
reported the rates of ACDRs related to anti-TB
therapy were approximately 4.8%-6% (Farazi et al.,
2014).
Male gender counts higher than female.
Some studies showed no differences between the
two genders in developing ADR to anti-TB therapy
(Sharma et al., 2002).We also found that ACDRs
appeared mostly in productive age (19-35 years).
Among all types of ACDRs, maculopapular rash
was the most common presentation, about almost
95% of all cases (Bigby, 2001).
In this study,
maculopapular rash also found to be the most
common ACDRs (66.7%). All types of anti-TB
therapy can induce maculopapular rash, even though
some studies reported it is most likely related to
pyrazinamide as the most offending drug, followed
by ethambutol, then isoniazid and rifampicin of all the
4 first-line anti-TB therapy (Tan et al., 2007).
However, in our study, patch testing that conducted
in 15 patients showed that rifampicin was the most
causative agents. Any possible explanation of this is
ACDRs may be associated with both type A and B
reaction, wherein the patch test indicates a
hypersensitivity reaction to a drug which is included
in type B reaction. Maculopapular rash is one of the
most common forms of allergic manifestations of
rifampicin, as well as urticaria and anaphylactic
reactions.
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
188

Stevens-Johnsons Syndrome (SJS) also found to
be a common manifestation of ACDRs in anti-TB
therapy recipients. Some studies reported cases of
SJS during the course of TB therapy, mostly caused
by rifampicin (Nyirenda & Gill, 1977). DRESS also
found in patients receiving anti-TB therapy according
to some reports. Anti-TB therapy that are known to
cause DRESS include isoniazid, rifampicin,
streptomycin, and pyrazinamide (Wang & Li, 2017).
Isoniazid was associated to occurrence of acneiform
eruption, as well as bullous drug reaction (Pantello &
Kondo, 2013). Exfoliative dermatitis and
erythroderma were least common manifestations to
anti-TB therapy, it was related to administration of
pyrazinamide and ethambutol (Jaisuresh, 2013).
Because of the limited type of effective anti-TB
therapy that can reach the favorable outcomes and
prevent TB relapse, correct assessment and
management of ACDRs to anti-TB therapy are
required. Rifampicin-based regimens are still
superior to non-rifampicin based until nowadays.
Rechallenge of anti-TB therapy by some steps of
desensitization should be considered in any condition
in which the advantages of TB therapy outweigh the
risk of possible reaction. Severe or life-threatening
history of ACDRs such as the bullous reactions,
erythroderma, DRESS, anaphylaxis, systemic
vasculitis and drug-induced autoimmune disease are
contraindicated to anti-TB therapy rechallenge and
therefore should be switched to alternative anti-TB
drug combination (Ton, 2008; Dheda, 2012).
5 CONCLUSION
Maculopapular rash is the most frequent type of
ACDRs induced by anti-TB therapy, while rifampicin
found to be the most frequent anti-TB therapy
inducing ACDRs according to patch test results in Dr.
Sardjito General Hospital Yogyakarta in 2014-2017.
REFERENCES
Bigby, M., 2001. Rates of cutaneous reactions to drugs.
Archives of dermatology 137, 765–70. doi:dea10005
[pii]
Departemen Kesehatan Republik Indonesia. 2016. Info
Datin: Tuberkulosis temukan obati sampai sembuh. 2–
10.
Farazi, A., Sofian, M., Jabbariasl, M., Keshavarz, S., 2014.
Adverse Reactions to Antituberculosis Drugs in Iranian
Tuberculosis Patients. Tuberculosis Research and
Treatment 2014, 1–6. doi:10.1155/2014/412893
Jaisuresh, K., 2013. Pyrazinamide-induced exfoliative
dermatitis in a patient on hemodialysis: a rare
complication. Case reports in nephrology 2013,
387293. doi:10.1155/2013/387293
Kardaun, S.H., Sekula, P., Valeyrie-Allanore, L., Liss, Y.,
Chu, C.Y., Creamer, D., Sidoroff, A., Naldi, L.,
Mockenhaupt, M., Roujeau, J.C., 2013. Drug reaction
with eosinophilia and systemic symptoms (DRESS):
An original multisystem adverse drug reaction. Results
from the prospective RegiSCAR study. British Journal
of Dermatology 169, 1071–1080.
doi:10.1111/bjd.12501
Lawrence Flick Memorial Tuberculosis Clinic. 1998.
Guidelines for the Management of Adverse Drug
Effects of Antimycobacterial Agents. 1–60.
Lehloenya, R.J., Dheda, K., 2012. Cutaneous adverse drug
reactions to anti-tuberculosis drugs: state of the art and
into the future. Expert Rev Anti Infect Ther 10, 475–
486. doi:10.1586/eri.12.13
Nyirenda, R., Gill, G.V. 1977. Stevens-Johnson syndrome
due to rifampicin. Br Med J ;2:1189.
Pontello Junior, R., Kondo, R.N., 2013. Drug-induced acne
and rose pearl: similarities. Anais Brasileiros de
Dermatologia 88, 1039–1040. doi:10.1590/abd1806-
4841.20132586
Sharma, S.K., Balamurugan, A., Saha, P.K., Pandey, R.M.,
Mehra, N.K., 2002. Evaluation of clinical and
immunogenetic risk factors for the development of
hepatotoxicity during antituberculosis treatment.
American Journal of Respiratory and Critical Care
Medicine 166, 916–919. doi:10.1164/rccm.2108091
Tan, W.C., Ong, C.K., Lo Rang, S.C., Abdul Razak, M.,
2007. Two years review of cutaneous adverse drug
reaction from first line anti-tuberculous drugs. Medical
Journal of Malaysia 62, 143–146.
Ton, Q. 2008. Management of common side effects of INH
( Isoniazid ), RIF ( Rifampin ), PZA ( Pyrazinamide ),
and EMB ( Ethambutol ). Univ South Nevada. 1–6.
Wang, L., Li, L.-F., 2017. Difficult clinical management of
antituberculosis DRESS syndrome complicated by
MRSA infection: A case report. Medicine 96, e6346.
doi:10.1097/MD.0000000000006346
World Health Organization. 2004. Treatment of
Tuberculosis: guidelines for national programmes -
Third edition. 1–55.
Adverse Cutaneous Drug Reactions Due to Antituberculosis Therapy in Dr. Sardjito General Hospital Yogyakarta
189
