
Drug Induced Hypersensitivity Syndrome (DIHS) Patient
Characteristics in Dermatology and Venereology Department, Dr.
Cipto Mangunkusumo National General Hospital in the Period 2014
to 2017
Teffy Nuary, Sarah Mahri, Windy Keumala B.
Deparment of Dermatology and Venereology Faculty of Medicine Universitas Indonesia/ Dr. Cipto Mangunkusumo
National General Hospital, Indonesia
Keywords: Drugs induced hypersensitivity, adverse drug reaction, characteristics, eosinofilia, culprit drugs.
Abstract: Drug induced hypersensitivity syndrome (DIHS) is a distinct, severe, idiosyncratic reaction to a drug
characterized by a prolonged latency period with cutaneous presentation and internal organ involvement. In
Asia, DIHS was reported to almost one tenth of adverse drug reaction cases, with a mortality rate ranged 3-
10%. The aim of this study are to describe the sociodemographic and clinical characteristics of patients with
DIHS and causative agents in Dermatology and Venereology Department Dr. Cipto Mangunkusumo National
General Hospital, Jakarta. This study is a descriptive study. Using medical records and electronic health
record of patients with DIHS were retrospectively reviewed. During 2014-2017 we identified 18 female, 14
male patients, with age range 14-87 years. Onset of the disease since exposed by culprit drugs were 14-40
days.The most common underlying disease was accute infection disease (43,7%). The DIHS clinical features
and laboratory finding in this study are fever (93,7%), maculopapular rash (90,6%), target lesions (12,5 %),
facial oedema (21,5%), periorbital oedema (12,5%). lymph nodes enlargement (93,7%), eosinofilia (31,2 %)
, elevating of liver function (100%), and one patients (3,1%) showed kidney involvement .The most common
causative drugs were antibacterials (60 %). all patients, the causative drug was discontinued and treated with
systemic corticosteroids. As the conclusion DIHS is a severe drug hypersensitivity reaction with prominent
cutaneous and systemic manifestations. Dispite the limitations, this study present some variations of DIHS
clinical features and many other drugs that implicated.
1 INTRODUCTION
Drug induced hypersensitivity syndrome (DIHS) is a
distinct, severe, idiosyncratic reaction to a drug
characterized by a prolonged latency period. It is a
life-threatening disease with cutaneous presentation
and internal organ involvement. Mechanisms that
have been implicated in DIHS include drug
detoxification enzyme abnormalities with subsequent
accumulation of reactive drug metabolites, sequential
reactivation of herpesviruses, such as
cytomegalovirus, Epsteine Barr virus, human
herpesvirus-6 and -7, and genetic predisposition
associated with certain human leukocyte antigen
alleles. DIHS clinical manifestations, usually fever,
rash, lymphadenopathy, eosinophilia, and a wide
range of mild-to-severe systemic presentations
(Husain et al, 2013). In Diagnose DIHS, clinicians
must exclude other potentially serious conditions,
including infections, neoplastic processes,
autoimmune disorders, and connective tissue disease.
Clinical testing and biopsy can be helpful, but are not
always specific (Husain et al, 2013) (Avancini et al,
2015). Scoring systems based on diagnostic criteria
have been developed by the European Registry of
Severe Cutaneous Adverse Reaction (RegSCAR):
acute rash, reaction suspected to be drug-related,
hospitalization, fever >38
o
,
enlarged lymph nodes
involving >2 sites, Involvement of > 1 internal organ,
blood count abnormalities, lymphocytes above or
below normal limits, platelets under normal limits
(Hiransuthikul et al, 2016).
In Asia, DIHS was reported to almost one tenth of
adverse drug reaction cases, with a mortality rate
ranged 3-10%. Mortality cases were mainly caused
by multiple organ failure and sepsis. Various
Nuary, T., Mahri, S. and B., W.
Drug Induced Hypersensitivity Syndrome (DIHS) Patient Characteristics in Dermatology and Venereology Department, Dr. Cipto Mangunkusumo National General Hospital in the Period
2014 to 2017.
DOI: 10.5220/0008152301270131
In Proceedings of the 23rd Regional Conference of Dermatology (RCD 2018), pages 127-131
ISBN: 978-989-758-494-7
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
127

medications have been described to be the cause of
DIHS (Hiransuthikul et al, 2016) (Chen et al, 2010).
The aims of this study are to describe the
sociodemographic and clinical characteristics of
patients with DIHS and causative agents in
Dermatology and Venereology Department dr. Cipto
Mangunkusumo Hospital, Jakarta within 2014 - 2017.
2 METHODS
This study is a descriptive retrospective study of
DIHS patients at dr. Cipto Mangunkusumo Hospital
between January 2014 and December 2017. The
medical records and electronic health record of
patients with DIHS were retrospectively reviewed.
The diagnostic criteria used in this study were
purposed by RegiSCAR. Hospitalization and reaction
suspected to be drug related were mandatory for
diagnosis. Also, 3 out of the following 7 criteria were
needed to fulfill the diagnosis: acute skin rash, fever
above 38
o
C, enlarged lymph node at 2 or more sites,
involved at least 1 internal organ, lymphocyte count
above or below laboratory limits, eosinophil count
above laboratory limits, and platelet count below
laboratory limits. This study has been approved by the
Health Research Ethical Committee of the Faculty of
Medicine, Universitas Indonesia.
3 RESULTS
A total of 32 medical records of DIHS patients in Dr.
Cipto Mangunkusumo Hospital in January 2014 –
December 2017 were reviewed in this study. This
study identified 18 females, 14 male patients, with
age range 14-87 years who fulfill the RegiSCAR
criteria. Onset of the disease since elicited by culprit
drugs 14-40 days. The most common underlying
disease was accute infection disease (43,7%). The
DIHS clinical features and laboratory finding in this
study are fever (93,7%), maculopapular rash (90,6%),
target lesions (12,5 %), facial oedema (21,5%),
periorbital oedema (12,5%). lymph nodes
enlargement (93,7%), eosinofilia (31,2 %), elevating
of liver function (100%) and one patient showed
kidney involvement (3,1%). (Table. 1). The most
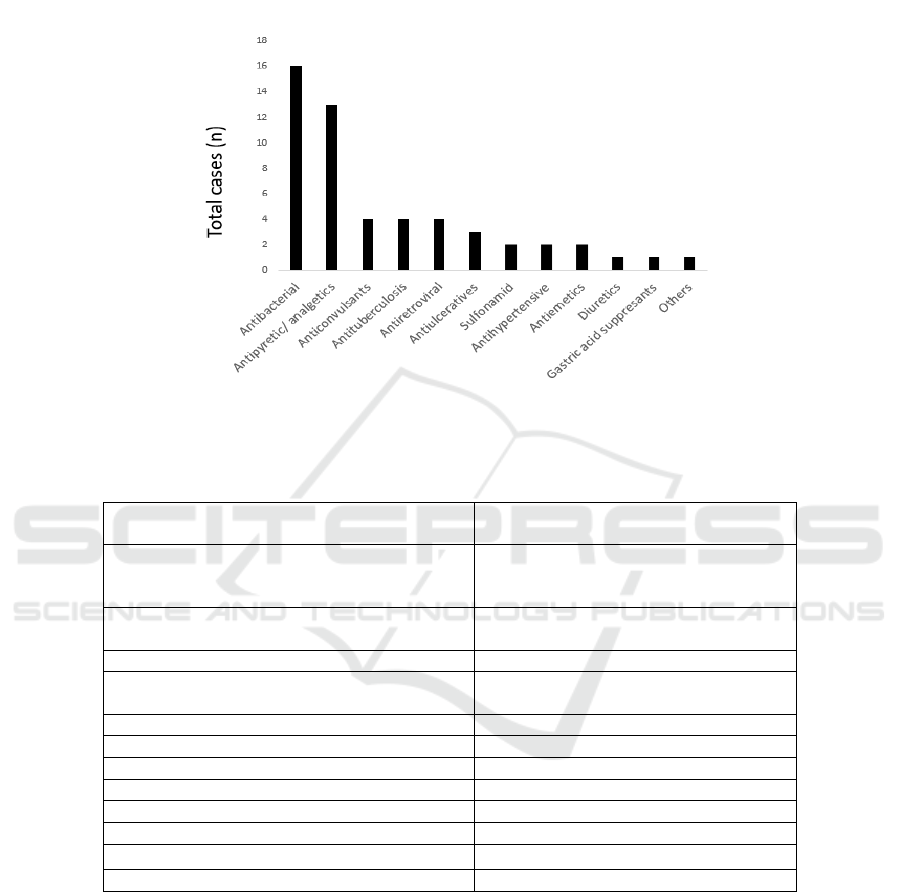
common causative drugs were antibacterials (60 %)
(Figure. 1, Table.2). The causative drug was
discontinued in all patients and treated with systemic
corticosteroids. There were four patients in this study
who had done the patch test. One patient had positive
result and relevan to rifampicin, dapsone,
paracetamol, klofazimin, and one patient had positive
result to rifampicin and isoniazid
. Two other
patients showed negative results.
4 DISCUSSION
DIHS is a rare, potentially life-threatening adverse
drug reaction with cutaneous manifestations and
internal organ involvement that occurs in both adults
and children. The latency period of DIHS is longer
than dose Steven - Johnson syndrome (SJS), Toxic
epidermal necrolysis (TEN, acute generelized
exanthematous pustulosis, fixed drug eruptions and
MPE, which all belong to delayed type
hypersensitivity (Shiohara et al, 2017). This study
showed the onset of DIHS were in range 14-40 days
after the start of eliciting drugs. Some study found
that longer period of latency may results in a failure
to properly make the diagnosis (Shiohara et al, 2017)
(Wang et al, 2017).
At the beginning, patients may expirience some
prodormal symptoms before or along with the
development of skin rash. These syptoms include
fever, pruritus, dysphagia, pain (Cho et al, 2017)
(Shiohara et al, 2017). There were 93,7% DIHS
patiens in this study had fever more than 38
0
C as
prodormal symptom. Although there can be various
cutaneous manifestations, in this study, almost all
patients had maculopapular (90,6%) rash, and some
patients had combination skin rash including,
maculopapular rash, target lesions, facial edema dan
periorbital edema. Akarin et al found. All DIHS
patients presented with rash, almost all were
maculopapular type (94.2%).
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
128
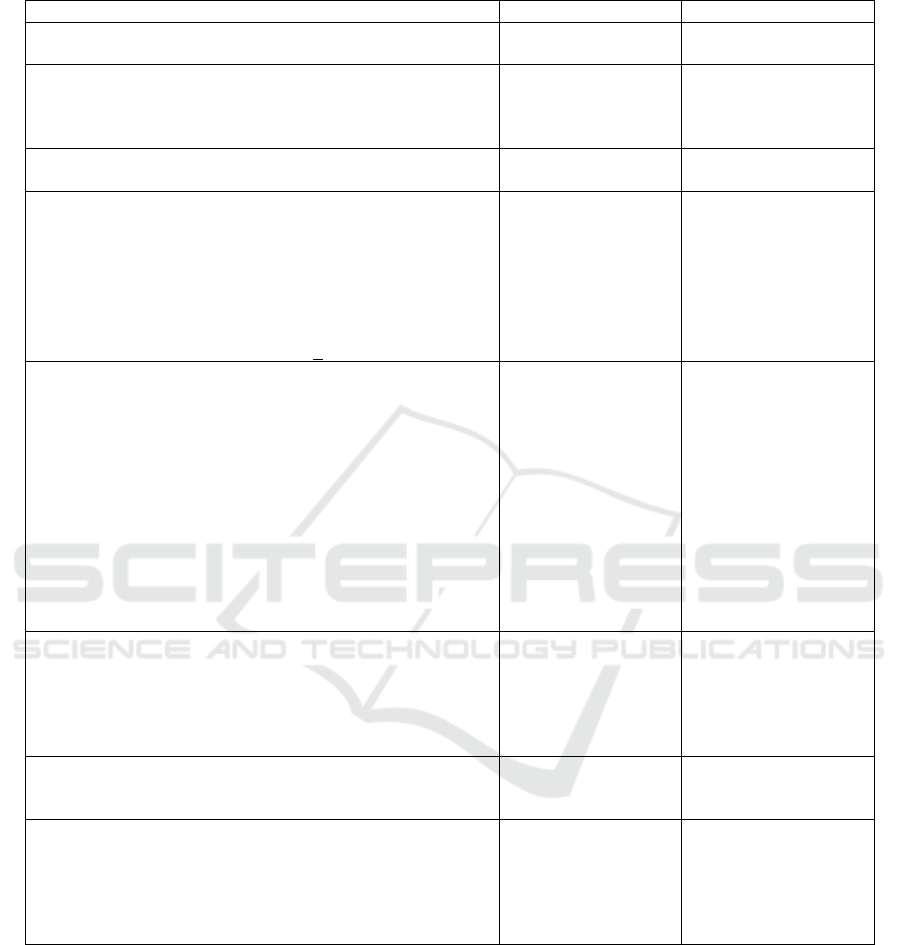
Table 1. DIHS patient characteristics in Department of Dermatology and Venereology, dr. Cipto Mangunkusumo Hospital
between January 2014 and December 2017.
n=32%
Age
43 (14-87) -
Sex
Male
Female
18
14
56,3
43.7
Onset (days)
18 (14-40) -
Clinical Symptoms
Skin Rash
Maculopapular Rash
Target Lesion
Facial Oedema
Periorbital Oedema
29
4
7
4
90,6
12.5
21.8
12.5
Fever >38
0
C 30 93.7
Enlar
g
ed l
y
m
p
h nodes involvin
g
> 2sites 30 93.7
Underlying disease
Accute Infection
Dermatomyositis
Tuberculosis
Convulsion Disorders
Hypothiroid
Leprosy
Cardiovascullar Disease (CVD)
Chronic Kidney Disease
Diabetes Melitus
Hypertension
Poliomiolitis
HIV
14
5
4
1
1
1
1
1
1
1
1
1
43,7
12.5
3.1
3.1
15.6
3.1
3.1
3.1
3.1
3.1
3.1
3.1
Internal Organ Involvement
Liver
Elevation of liver function
AST (U/L)
ALT (U/L)
Kidney
32
165 (42-654)
175 (45-483)
1
100
-
-
3,1
Eosinofil
Eosinophilia
Eosinophil level ove
r
laboratory limits (µL)
10
756 (501-6730)
31.2
-
Therapy
Corticosteroid (methylprednisolon)
1 mg/Kg
Length of Stay (days)
1,5 mg/Kg
Len
g
th of Sta
y
(
da
y
s
)
18
8 (6-15)
14
9
(
6-23
)
56.2
43.8
There were 93,7 % patients who showed enlarged
of lymph nodes involving two sites or more in this
study. Akarin et al and Prannee et al, showed
patients may have limited lymph node involvement
or generalized. lymphadenopathy with localized
tenderness involving the cervical, axillary, and
inguinal lymph nodes. In approximately 31,2% of
cases in this study, there is eosinphilia With > 5.0x
10
9
eosinophils/L. Eosiophilia can be delayed for 1
to 2 weeks. Hypereosinophilia likely plays a role in
visceral manifestations because eonsinophil granule
proteins are toxic to many tissues (Wang et al,
2017). The liver is the most frequently affected
visceral organ in DIHS, oftennwith varying degrees
of hepatitis (Wongkitisophon et al, 2012). All
patients in this study had elevating of liver function.
Stander et al, found there were Phenytoin,
minocycline, and dapsone are commonly
implicated. The elevated liver enzymes may persist
for several days after withdrawal of culprit drug, but
Drug Induced Hypersensitivity Syndrome (DIHS) Patient Characteristics in Dermatology and Venereology Department, Dr. Cipto
Mangunkusumo National General Hospital in the Period 2014 to 2017
129
may sometimes take months to completely resolve
(Wongkitisophon et al, 2012) (Shiohara et al, 2006).
There were one patients showed kidney
involvement that presented by increasing of urea
and creatinin. There were no lung and heart
alterations. Li Wang et al presented that damage
occurred most commonly to the liver, followed by
the kidneys.
Figure 1. Culprit drugs
Table 2. Culprit drug details of the DIHS in Department of Dermatology and Venereology, dr. Cipto Mangunkusumo
Hospital between January 2014 and December 2017
Drug Categories Drug Names*
Antibacterial Cefadroxil, ceftriaxon, cefixim,
tiamfenicole, ciprofloxacin, ofloxacin,
metronidazole, mero
p
ene
m
Antipyretic/ Analgetic Paracetamol, tramadol, ibuprofen,
metampiron, mefinamic aci
d
Anticonvulsant Carbamaze
p
in, halo
p
eridol, Phen
y
toin
Antituberculosis Rifampicin, isoniazid, ethambutol,
p
irazinami
d
Antiretroviral Lamivudine, zidovudine, nevirapine
Antiulcerative Ome
p
razole, lanso
p
razole
Sulfonami
d
Da
p
sone
Antih
yp
ertensive Amlodi
p
ine, nifedi
p
ine
Antiemetic Ondancentron, domperidone
Diuretic Furosemide
Gastric acid suppressant Ranitidine
Others Pet tze huang
*The drug names are arranged respectively
Many drugs have been reported to be a causative
agent of DIHS. However, only a limited number of
drugs are frequently encountered as culprits,
including anti-convulsants, antibacterials
,antivirals, antipyretics, and others (Wang et al,
2017) (Stander et al, 2013). The most peculiar
feature of these culprits is a long latent period,
which ranges from 3 to 8 weeks after
commencement of the drugs (Shiohara et al, 2017).
This study present the most common culprit drugs
were antibacterials (cefadroxil, ceftriaxon, cefixim,
tiamfenicole, ciprofloxacin, ofloxacin,
metronidazole, meropenem, respectively) that
might be associated with the most common patients
underlying disease in this study were accute
infection diseases (43,7%) including acute
respiratory tract, urinary tract infections, and other
secondary infection.
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
130

In all patients, the causative drug was
discontinued. All patiens were treated with systemic
corticosteroids, eighteen patients got 1mg/kg body
weight and fourteen patients got 1,5 mg/kg weight
methylprednisolon as initial dose. The state of the
patients was a consideration to determine the initial
dose. This study showed patient who got higher
initial dose had longer length of stay, patient
underlying disease were thought as the factors that
contributing. In this study just four patients who
underwent patch test. Some difficulties were
thought due to DIHS latency and patients
complience.
Study limitations include a retrospective study,
small number of subjects. More epidemiology study
to confirm and provide more useful clinical
information for early detection and improve the
outcome of severe cutaneous adverse reactions
Including DIHS is needed.
5 CONCLUSION
DIHS is a severe drug hypersensitivity reaction with
prominent cutaneous and systemic manifestations.
Dispite the limitations, this study presents some
variations of DIHS clinical features. Although it is
classically caused by anticonvulsants and
sulfonamides, many other drugs have been
implicated, such as antibiotics. More larger
epidemiology study either retrospective or
prospective are needed to provide more useful
clinical informations.
ACKNOWLEDGEMENT
Thanks to medical record staffs helping, for data
collecting.
REFERENCES
Avancini, J., Maragno, L., Santi, C. G., & Criado, P. R.,
2015. Drug reaction with eosinophilia and systemic
symptoms/drug‐induced hypersensitivity syndrome:
clinical features of 27 patients. Clinical and
experimental dermatology, 40(8), pp. 851-859.
Chen, Y. C., Chiu, H. C., & Chu, C. Y., 2010. Drug
reaction with eosinophilia and systemic symptoms: a
retrospective study of 60 cases. Archives of
dermatology, 146(12), pp. 1373-1379.
Cho, Y. T., Yang, C. W., & Chu, C. Y. (2017). Drug
reaction with eosinophilia and systemic symptoms
(DRESS): an interplay among drugs, viruses, and
immune system. International journal of molecular
sciences, 18(6), pp. 1243.
Hiransuthikul, A., Rattananupong, T., Klaewsongkram,
J., Rerknimitr, P., Pongprutthipan, M., &
Ruxrungtham, K., 2016. Drug-induced
hypersensitivity syndrome/drug reaction with
eosinophilia and systemic symptoms
(DIHS/DRESS): 11 years retrospective study in
Thailand. Allergology International, 65(4), pp. 432-
438.
Husain, Z., Reddy, B. Y., & Schwartz, R. A., 2013.
DRESS syndrome: Part I. Clinical
perspectives. Journal of the American Academy of
Dermatology, 68(5), 693-e1.
Shiohara T, Inaoka M, Kano Y. Drug‑induced
hypersensitivity syndrome (DIHS): A reaction
induced by a complex interplay among herpesviruses
and antiviral and antidrug immune responses.
Allergol Int 2006;55:1‑8. doi:
10.2332/allergolint.55.1.
Shiohara, T., & Kano ,Y., 2017. Drug reaction with
eosinophilia and systemic symptoms (DRESS):
Incidence, pathogenesis and management. Expert
Opin Drug Safety, 16, pp. 139‑147. doi:
10.1080/14740338.2017.1270940.
Ständer, S., Metze, D., Luger, T., Schwarz, T., 2013.
Drug Reaction with Eosinophilia and Systemic
Symptoms (DRESS): Eine Übersicht. Hautarzt,
64(8), pp. 611–624.
Wang, L., & Mei, X. L., 2017. Drug Reaction with
Eosinophilia and Systemic Symptoms: Retrospective
Analysis of 104 Cases over One Decade. Chinese
medical journal, 130(8), pp. 943-949.
Wongkitisophon, P., Chanprapaph, K.,
Rattanakaemakorn, P., & Vachiramon, V., 2012. Six-
year retrospective review of drug reaction with
eosinophilia and systemic symptoms. Acta dermato-
venereologica, 92(2), pp. 200-205.
Cacoub, P., Musette, P., Descamps, V., Meyer, O., Speirs,
C., Finzi, L., & Roujeau, J. C., 2011. The DRESS
syndrome: a literature review. The American journal
of medicine, 124(7), pp. 588-597, doi:
10.1016/j.amjmed.2011.01.017.
Drug Induced Hypersensitivity Syndrome (DIHS) Patient Characteristics in Dermatology and Venereology Department, Dr. Cipto
Mangunkusumo National General Hospital in the Period 2014 to 2017
131
