
A Four Years Retrospective Study of Stevens Johnson Syndrome:
Toxic Epidermal Necrolysis Treatments in a National Tertiary
Referral Hospital
Sarah Mahri, Teffy Nuary, Fadhli A. Mughni, and Windy Keumala B.
Department of Dermatology and Venereology, Faculty of Medicine Universitas Indonesia / Dr. Cipto Mangunkusumo
National General Hospital, Jakarta
Keywords: Stevens-Johnson Syndrome, Toxic Epidermal Necrolysis, Treatments
Abstract: Stevens-Johnson syndrome (SJS) and/or toxic epidermal necrolysis (TEN) are drug reactions associated to
high morbidity and mortality. Prompt diagnosis and management may reduce the mortality rate. The research
aims to evaluate the consistency of current treatments for SJS/TEN with the clinical pathway by Dr. Cipto
Mangunkusumo National General Hospital and Indonesian Society of Dermatology And Venereology
(ISDV). A retrospective review was conducted on patients with SJS/TEN admitted to Dr. Cipto
Mangunkusumo National General Hospital, Jakarta during January 2014 to December 2017. The data were
collected from paper-based and electronic health medical record database. A total of 34 cases of SJS/TEN
were admitted, but only 30 cases with complete data was included, comprising of 20 males and 10 females
with the mean age were 37.5 (15-70) years. Carbamazepin was the most common culprit drug. All patients
were treated with intravenous methylprednisolone. The average length of stay were 6 days (3-20) in SJS, 8
(3-18) in SJS-TEN, and 11 (4-18) in TEN, while the mortality rate were 18.2% in SJS, 8.3% in SJS-TEN, and
14.3% in TEN. As conclusion, corticosteroids may contribute to reduced mortality rate in SJS/TEN without
increasing secondary infection and serious sequele. The current treatments for SJS/TEN in our hospital is
consistent with the clinical pathway by Dr. Cipto Mangunkusumo National General Hospital and Indonesian
Society of Dermatology And Venereology (ISDV). Further well-designed studies are required to compare the
effect of corticosteroids treatment for SJS/TEN to other medications.
1 INTRODUCTION
Stevens-Johnson syndrome (SJS) and toxic epidermal
necrolysis (TEN) are life-threatening diseases
characterized by widespread red rash, blisters, and
shedding of dead skin, with mucosal involvement.
The incidence of SJS/TEN has been reported to be
1.5–1.8/per million persons per year. SJS and TEN,
based on clinical manifestations, are generally
considered as different spectrum of the disease.
Bastuji-Garin et al. proposed
that disease
classification should be based on the percentage
of the
total body surface area (BSA) of the epidermolysis or
epidermal detachment. Epidermal detachment <10%
of the BSA is classed as SJS, detachment above 30%
as TEN, and detachment between 10% and 30% as
intermediate (SJS/TEN overlap). SJS/TEN usually
caused by medications (Wang & Mei, 2017; Bastuji
et al., 1993).
In addition to the damage to the skin,
gastrointestinal tract, and respiratory tract mucosa,
SJS/TEN can also associated with visceral
involvement (e.g liver, kidneys, lungs, and
hematopoietic system), leading to organ dysfunction
or even failure. The mortality rates of SJS and TEN
are 10% and 34%, respectively (Kim et al., 2012; Lee
et al., 2011).
SCORTEN, a severity-of illness scoring system
for TEN was used to evaluate prognosis. The
SCORTEN criteria are: serum blood urea nitrogen
>10 mmol/L, serum bicarbonate <20 mmol/L, serum
glucose >14 mmol/L, age >40 years, malignancy
present, heart rate >120 bpm, and percentage of BSA
with epidermal detachment >10%. The mortality rate
was predicted according to the SCORTEN total score
as follow: 1 point, 3.2%; 2 points, 12.1%; 3 points,
35.3%; 4 points, 58.3%; and 5 or more points, 90%
(Fouchard et al., 2000).
Mahri, S., Nuary, T., Mughni, F. and B., W.
A Four Years Retrospective Study of Stevens Johnson Syndrome: Toxic Epidermal Necrolysis Treatments in a National Tertiary Referral Hospital.
DOI: 10.5220/0008151300830087
In Proceedings of the 23rd Regional Conference of Dermatology (RCD 2018), pages 83-87
ISBN: 978-989-758-494-7
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
83

The treatment for these diseases is not well
established all over the world. Clinical pathway 2017
for SJS/TEN treatments by Indonesian Society of
Dermatology And Venereology (ISDV) include
discontinue potential offending drugs,
hospitalization, ophthalmologist consultation,
systemic corticosteroids: intravenous dexamethasone
prednisone equivalent dose 1-4 mg/kg/day for SJS, 3-
4 mg/kg/day for SJS-TEN, and 4-6 mg/kg/day
intravenous; IVIG high dose 1 g/kg/day for 3 days in
TEN; cyclosporine; and combination IVIG and
systemic corticosteroids, and topical treatment
include petrolatum gel with parafin liquid or
debridement. In addition to clinical pathway by
ISDV, clinical pathway by Dr. Cipto Mangunkusumo
National General Hospital for the treatment of
SJS/TEN include identify and discontinue potential
offending medications/drugs and other drugs that can
cross react, hospitalization, intravenous fluid drug,
systemic corticosteroids: intravenous
methylprednisolone (prednisone equivalent dose) 1-2
mg/kg/day for SJS; 2-3 mg/kg/day for SJS-TEN; and
3-4 mg/kg/day for TEN, topical treatment for erosion
with 1% salicylic acid in cream or vaselin album or
fucidic acid cream 2%, Nacl 0.9% for crusts lesions,
and consultations to ophthalmologist; dentists,
internist; and otolaryngologist.
We conducted a retrospective review on patients
admitted to Dr. Cipto Mangunkusumo National
General Hospital, Jakarta with a diagnosis of SJS,
SJS-TEN overlap and TEN based on clinical features
during four years. The aim of this study is to evaluate
the consistency of SJS/TEN current treatments with
the clinical pathway by Dr. Cipto Mangunkusumo
National General Hospital and Indonesian Society of
Dermatology And Venereology (ISDV).
2 METHODS
A retrospective review was performed on patients
admitted to Dr. Cipto Mangunkusumo National
General Hospital, Jakarta, with the diagnosis of
SJS/TEN based on clinical features. The data were
collected from paper-based and electronic health
medical record database from January 2014 to
December 2017. Diagnostic criteria were based on
those proposed by Bastuji-Garin et al (Bastuji et al.,
1993). Prognostic were assessed using the
SCORTEN standard system. (Bastuji et al., 1993;
Kim et al., 2012). The following datas were collected:
demographic information, time from onset to
admission, culprit drugs, underlying diseases,
SCORTEN, extent of mucocutaneous involvement,
laboratory data, treatments, complications, and
mortality.
Institutional ethical committee clearance was
obtained. All drugs that have been taken within six
weeks before the onset of symptoms were considered
as the culprit drugs (Yamane et al., 2007).
3 RESULTS
Of the total 34 medical records, 30 with complete data
were selected and four with uncomplete data were
excluded. The clinical characteristics of patients are
available in table 1.
In our study, drug hypersensitivity was the causes
in all SJS/TEN patients. The causative drugs are
shown in figure 1. The most common culprit drug was
anticonvulsants (carbamazepine, fenobarbital,
haloperidol, gabapentin, pregabalin, lamotrigin,
fenitoin, valproic acid), followed by antibiotics
(cefixime, cotrimoksazole, ciprofloxacin, cefadroxil,
meropenem levofloxacin, clindamicyn, amoxicillin),
NSAIDs (paracetamole, mefenamic acid, ibuprofen,
metamphyron), antituberculosis (rifampicin,
isoniazide, pirazinamide, ethambutol), antiretroviral
(tenofovir, nevirapine, stavudine, lamivudine),
antiulcerative (ranitidin, omeprazole, lansoprazole),
cough and flu medicines (ephedrine,
phenylpropanolamine, phenylephrine, bromhexine,
N-acetylcysteine), tramadol, antigout (allopurinol),
antihistamine (cetirizine), anticancer (5-fu, cisplatin),
antihypertension (captopril) , anticoagulant
(transamin, aspilet), furosemide, antiparasites
(pirimetamine, rescovulin), antiemetics (ondansetron,
metoclopramide) and other drugs (activated
attapulgite, loperamide, eperisone, fructus
schizandrae extract).
Laboratory abnormalities showed increased
amino transferase (AST, ALT), hiponatremia,
anemia, leucocytosis, azotemia, hypoalbuminemia,
thrombocytopenia, hyperglycemia, and increased
procalcitonin. Patch test was performed in two
patients, one patient had positive patch test for
carbamazepine, and the other patient showed negative
result.
All 30 cases (100%) were treated with intravenous
methylprednisolone and fast tappering to oral
methylprednisolone. Corticosteroids usage, length of
stay, and mortality rate are shown in table 2.
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
84

Table 1. Clinical characteristics of SJS/TEN patients (n=30)
n (%)
Age (years) 37.50 (15-70)
Gender
Male
Female
20 (66.7)
10 (33.3)
Time from onset to
admission (days)
3 (1-9)
Diagnosis
SJS
SJS-TEN
TEN
11 (36.7)
12 (40.0)
7 (23.3)
Underlying diseases
Epilepsi
Gastrointestinal
problem
HIV infection
Chronic Kidney
Disease
Hyperuricemia
Cardiovascular
Disease
Respiratory Tract
Infections
Stroke
Tuberculosis
Malignancy
Diabetes mellitus
Systemic Lupus
Erythematosus
Hepatitis
9 (30.0)
7 (23.3)
6 (20.0)
5 (16.7)
5 (16.7)
5 (16.7)
4 (13.3)
3 (10.0)
3 (10.0)
3 (10.0)
2 (6.7)
2 (6.7)
1 (3.3)
Mucosal involvement
Oral
Ocular
Genitalia
28 (93.3)
21 (70.0)
11 (36.7)
SCORTEN
≤1
2
3
4
≥5
3 (10.0)
8 (26.7)
11 (36.7)
5 (16.7)
1 (3.0)
Organ involvement and
complications
Liver dysfunction
Renal
dysfunction
Pulmonary
infections
Sepsis
Electrolyte
imbalance
9 (30)
6 (20)
3 (10)
6 (20)
8 (26)
Figure 1. The causative drugs of SJS/TEN
A Four Years Retrospective Study of Stevens Johnson Syndrome: Toxic Epidermal Necrolysis Treatments in a National Tertiary Referral
Hospital
85
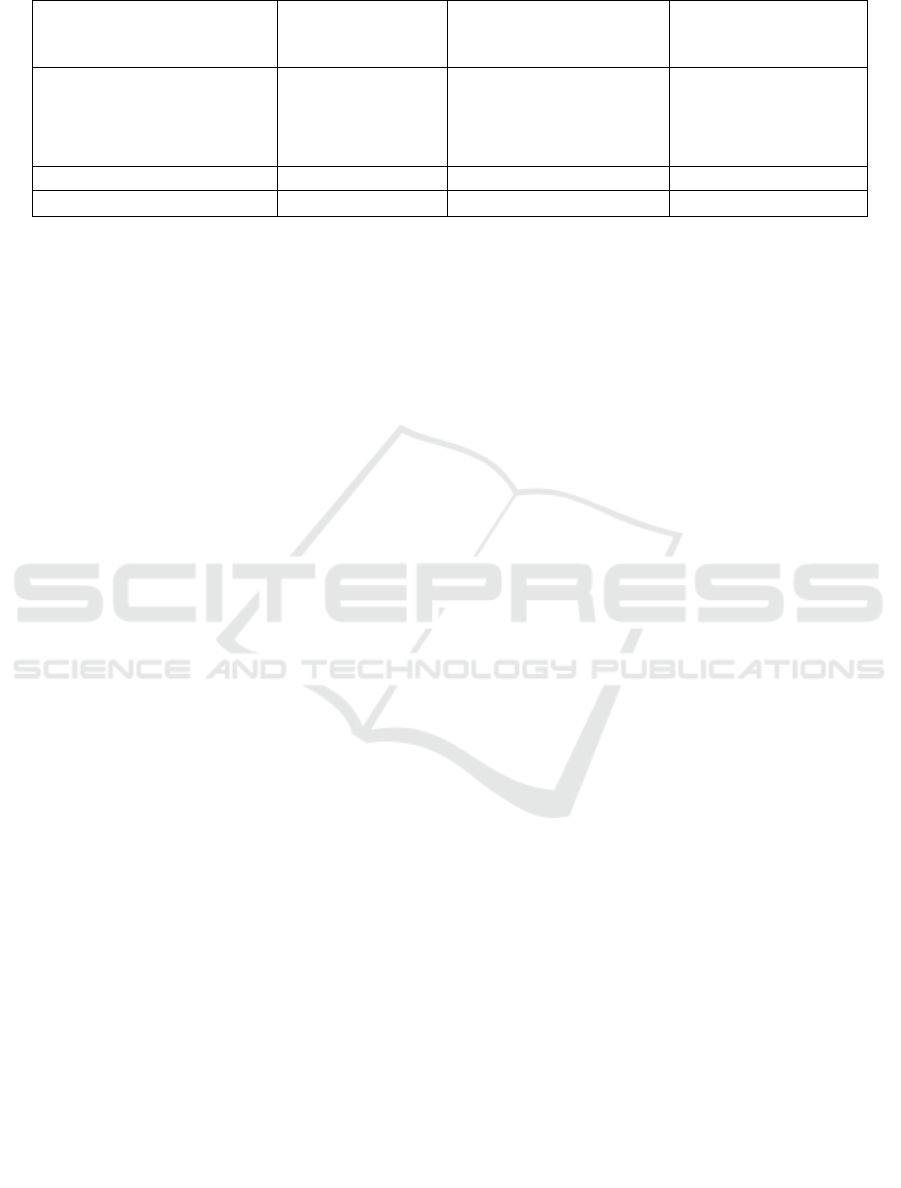
Table. 2 Corticosteroids doses, length of stay and mortality in 30 patients
SJS (n=11)
n (%)
SJS-TEN overlap
(n=12)
n (%)
TEN (n=7)
n (%)
Corticosteroids
1 mg/kg/day
1.5 mg/kg/day
2 mg/kg/day
9 (81.8)
2 (18.2)
0 (0)
0 (0)
9 (75)
3 (25)
0 (0)
2 (28.6)
5 (71.4)
Length of stay (days) 6 (3-20) 8 (3-18) 11 (4-18)
Mortality rate 2 (18.2) 1 (8.3) 1 (14.3)
4 DISCUSSION
The median age was 37.5 years, which is lower than
those reported from other countries in Asia such as
Bangkok, Japan, Singapore, and Korea (Tan & Tay,
2012; Kim et al., 2012).
Our study shows that females
are affected with SJS/TEN more than males with a
male-to-female ratio of 2:1, which was in agreement
with earlier studies (Yamane et al., 2007; Kim et al.,
2012). The causes of SJS/TEN were considered to be
caused by an adverse reaction to drugs. The most
common culprit drug was anticonvulsants, especially
carbamazepine, followed by antibiotics, and
NSAIDs, this is in agreement with study by Yamane
et al
6
. The results may be because antibiotics and
NSAIDs were available without prescription in
Indonesia, and thus many patients with fever,
headache, or other symptoms of infection purchased
such drugs over the counter instead of consulting a
doctor.
All 30 cases (100%) in our study were treated with
intravenous corticosteroids, methylprednisolone.
Methylprednisolone for SJS was 1-1.5 mg/kg/day
prednisone equivalent dose. In SJS-TEN overlap and
TEN group, the dose was increased to 1.5-2
mg/kg/day prednisone equivalent dose. These
treatment is consistent with clinical guidelines for
SJS/TEN treatment by Dr. Cipto Mangunkusumo
National General Hospital and Indonesian Society of
Dermatology And Venereology (ISDV) that
recommends application of systemic corticosteroids
1-4 mg/kg/day. The differences between clinical
guidelines from ISDV and our hospital is the
preferences to use methylprednisolone rather than
dexamethasone, because of the minimal adverse
reactions. The corticosteroids doses were in
agreement with guidelines by Gupta LK that
recommends prompt withdrawal of the culprit drug,
meticulous supportive care, and early (preferably
within 72 hours) initiation of moderate to high dose
of oral or parenteral corticosteroid (prednisolone 1-2
mg/kg/day or equivalent), tapered rapidly within 7-10
days (Gupta et al., 2016).
Additional therapies include supportive care with
intravenous fluid drug, systemic antibiotics for
prophylaxis, skin and wound care with 1% salicylic
acid in cream or petrolatum and topical antibiotics for
the secondary infections. No patient received other
immunosuppressant or intravenous immunoglobulin.
All patients were consulted to ophtamologist, dentist,
otolaryngologist, and internist, consistent ith clinical
pathway.
The length of stay in TEN group is higher than
SJS and SJS-TEN overlap group. A total four cases
died, two cases in SJS group, one case in SJS-TEN
overlap, and one case in TEN group. Among SJS
group, one patient both of patients who died had a
severe underlying disease, one patient with end stage
renal failure had complications such as septic shock
and pneumonia, the other patient had a carcinoma
sinonasal. The actual mortality rate in SJS group was
higher (18.2%) compared to the predicted mortality
rate (12.1%). The differences underlying diseases and
infectious morbidity mainly influenced the mortality
rate. In the SJS-TEN overlap group, one case with
SCORTEN 4 died due to septic shock. The actual
mortality rate in SJS-TEN overlap is lower (8.3%)
than the predicted mortality rate (35.3%). One patient
who died in TEN group was a 18 years old female
with systemic lupus erythematosus, end stage renal
failure, epilepsi, hypertension, ischemic heart disease
and complications such us pneumonia and pleural
efusion. The actual mortality rate in TEN group
(14.3%) was lower than the predicted mortality score
(35.3%).
5 CONCLUSIONS
Corticosteroids moderate or high doses and short
period may contribute to reduced mortality rate in
SJS/TEN without increasing secondary infection and
serious sequele. The current treatments for SJS/TEN
RCD 2018 - The 23rd Regional Conference of Dermatology 2018
86

in our hospital is consistent with the clinical pathway
an clinical guidelines by Dr. Cipto Mangunkusumo
National General Hospital and Indonesian Society of
Dermatology and Venereology (ISDV). Further well-
designed studies are required to compare the effect of
corticosteroids treatment for SJS and/or TEN.
ACKNOWLEDGEMENTS
We express our gratitude to Dr. Cipto
Mangunkusumo National General Hospital medical
record staff for data collection.
REFERENCES
Bastuji-Garin, S., Rzany, B., Stern, R.S., Shear, N.H.,
Naldi, L. and Roujeau, J.C., 1993. Clinical
classification of cases of toxic epidermal necrolysis,
Stevens-Johnson syndrome, and erythema
multiforme. Archives of dermatology, 129(1), pp.92-
96.
Fouchard, N., Bertocchi, M., Roujeau, J.C., Revuz, J.,
Wolkenstein, P. and Bastuji-Garin, S., 2000.
SCORTEN: a severity-of-illness score for toxic
epidermal necrolysis. Journal of Investigative
Dermatology, 115(2), pp.149-153.
Gupta, L.K., Martin, A.M., Agarwal, N., D’Souza, P., Das,
S., Kumar, R., Pande, S., Das, N.K., Kumaresan, M.,
Kumar, P. and Garg, A., 2016. Guidelines for the
management of Stevens–Johnson syndrome/toxic
epidermal necrolysis: An Indian perspective. Indian
Journal of Dermatology, Venereology, and
Leprology, 82(6), p.603.
Kim, H.I., Kim, S.W., Park, G.Y., Kwon, E.G., Kim, H.H.,
Jeong, J.Y., Chang, H.H., Lee, J.M. and Kim, N.S.,
2012. Causes and treatment outcomes of Stevens-
Johnson syndrome and toxic epidermal necrolysis in 82
adult patients. The Korean journal of internal
medicine, 27(2), p.203.
Lee, H.Y., Tey, H.L., Pang, S.M. and Thirumoorthy, T.,
2011. Systemic lupus erythematosus presenting as
Stevens–Johnson syndrome and toxic epidermal
necrolysis: a report of three cases. Lupus, 20(6),
pp.647-652.
Tan, S.K. and Tay, Y.K., 2012. Profile and pattern of
Stevens-Johnson syndrome and toxic epidermal
necrolysis in a general hospital in Singapore: treatment
outcomes. Acta dermato-venereologica, 92(1), pp.62-
66.
Tripathi, A., Ditto, A.M., Grammer, L.C., Greenberger,
P.A., McGrath, K.G., Zeiss, C.R. and Patterson, R.,
2000, March. Corticosteroid therapy in an additional 13
cases of Stevens-Johnson syndrome: a total series of 67
cases. In Allergy and asthma proceedings (Vol. 21, No.
2, p. 101). OceanSide Publications.
Wang, L. and Mei, X.L., 2017. Retrospective analysis of
stevens-johnson syndrome and toxic epidermal
necrolysis in 88 Chinese patients. Chinese medical
journal, 130(9), p.1062.
Yamane, Y., Aihara, M. and Ikezawa, Z., 2007. Analysis of
Stevens-Johnson syndrome and toxic epidermal
necrolysis in Japan from 2000 to 2006. Allergology
International, 56(4), pp.419-425.
A Four Years Retrospective Study of Stevens Johnson Syndrome: Toxic Epidermal Necrolysis Treatments in a National Tertiary Referral
Hospital
87
