
Absorption of NO
2
by Sodium Sulfite Solution Adding
Ethanol in High Oxygen Concentration
P Wang
1
, L N Wu
1,*
, L Cui
1
and T Fan
2
1
National Engineering Lab for Coal-fired Pollutants Emission Reduction, Shandong
University, Jinan 250061, China
2
Shengli Youtian Kangbei Shiyou Gongcheng Zhuangbei Limited company,
DongYing 257000 China
Corresponding author and e-mail: L N Wu, 201512938@mail.sdu.edu.cn
Abstract. In order to achieve effective removal of NO
2
by sodium sulfite solution with a high
oxygen concentration, an additive was explored in this study. Flue gas was oxidized by ozone
and was then absorbed by sodium sulfite solution adding ethanol into with a high oxygen
concentration. Research showed that sodium sulfite solution, with the addition of ethanol, was
an effective absorbent in high oxygen concentrations. Influencing factors such as the type of
additive, added ethanol concentration, absorption temperature, absorption product, and
enrichment of ion (NO
2
-
and NO
3
-
), were investigated during the absorption process of the
NO
2
by sodium sulfite solution. The optimal additive was ethanol in the removal of NO2 by
sodium sulfite solution. In regard to the absorption product, NO
2
-
and NO
3
-
were reaction
products, which decreased absorption efficiency of NO
2
. Furthermore, increase of ethanol
concentration in sodium sulfite solution could counteract adverse effects of ion during the
absorption of the NO
2
.
1. Introduction
A large number of pollutants are produced in the production of steel, which have caused serious
environmental problems. Flue gas pollutants produced by sintering processes account for 55% of
these pollutants, [1] which are from unstable, hyperoxic (15%), [2] low temperature (150 °C) flue gas.
A draft of advice for emission standards from EPD of China was issued, in which NO
x
emission
concentration was modified from 300 mg/m
3
to 100 mg/m
3
. At present, denitrification technology
mainly includes SCR, SNCR, and activated carbon adsorption and ozone oxidation wet absorption
combined with ozone oxidation [3] which are easily adapted and developed.
Wet absorption combined with oxidation technology consists of two parts: namely oxidation and
absorption. This technology has been studied by scholars at home and abroad. Researched showed
that the main product of oxide of NO by ozone is NO
2
. [4]
Wet absorption is mainly aimed at NO
2
whose solubility is much greater than that of NO. Zhuang [5] reported that pH had a significant
influence on the absorption of SO
2
and NO
2
in alkali solution. When pH is higher than 6, NO
2
absorption efficiency could reach 80%. Guo et al. [6] determined that (NH
4
)
2
SO
3
formed in ammonia
desulphurization was the effective component of denitrification. The process of denitrification is
actually that of oxidation-reduction between SO
3
2-
and NO
2
. Sun [7]
et al. found that MgO was an
424
Wang, P., Wu, L., Cui, L. and Fan, T.
Absorption of NO2 by Sodium Sulfite Solution Adding Ethanol in High Oxygen Concentration.
In Proceedings of the International Workshop on Environmental Management, Science and Engineering (IWEMSE 2018), pages 424-430
ISBN: 978-989-758-344-5
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
effective NO
2
absorbent. Improvement of pH and MgO concentration promoted NO
2
absorption
efficiency. Chen L et al. used Na
2
SO
3
as an NO
2
absorbent whose concentration had a significant
influence on NO
2
absorption efficiency. In this research the ratio of liquid to gas became a dominant
factor of NO
2
absorption. NO
2
absorption efficiency is 90% when the liquid to gas ratio equaled to 4,
while the efficiency is 55% when ratio is 1.8. [8]
Tang [9] et al. reported that Na
2
SO
3
and CaSO
3
were effective absorbents. The absorption
efficiency and consumption of Na
2
SO
3
was much greater than those of CaSO
3
. These studies had
indicated that SO
3
2-
played an important role in the absorption of NO
2
.
In previous researches oxygen concentration of flue gas was lower (6%) which was not in
accordance with characteristics of sintering flue gas. In this paper exploratory research on absorption
of NO
2
by sodium sulfite adding ethanol in high oxygen concentration (15%) was investigated.
2. Experimental section
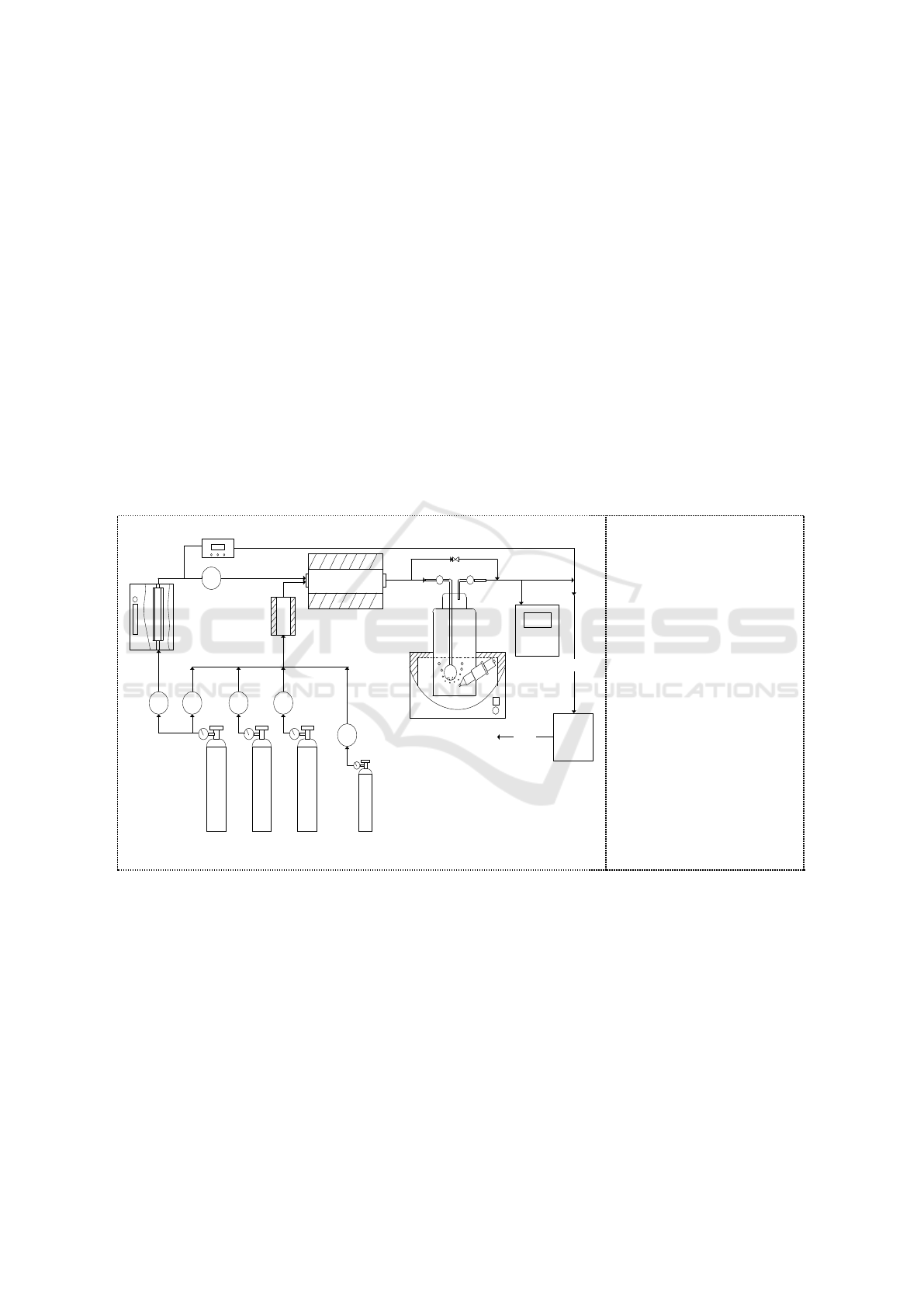
2.1. Experimental System.
A schematic diagram of the experimental setup for the bubble column reactor absorption of NO
2
generated in oxidation part was shown in Figure 1. The experimental system is divided into three
parts namely, gas phase oxidation, bubble column reactor absorption and gas detection.
M
M
M
MMM
1
2
3 4 5 6
7
8
9
10
11
12
13
Clear
Exhaust
14
Figure 1. Schematic
diagram of the
experimental apparatus.
1. Ozone generator
2. Mass flow controllers
3. O
2
Cylinder
4. N
2
cylinder
5. CO
2
cylinder
6. NO cylinder
7. Ozone concentration
detector 8. Reheating
mixer
9. Oxidation tube
10. Bubbling reactor
11. pH meter
12. Gas analyzer
13. Tail gas treatment
device 14. Water bath.
The flue gas in the laboratory came from the gas cylinder (CO
2
N
2
O
2
NO) controlled by mass
flow controllers. The initial concentration was set to be 4%, 15% and 200mg/m
3
for CO
2
, O
2
,and NO
respectively. The simulated flue gas was mixed and preheated in a preheating mixer at a temperature
of 150°C . Then, flue gas was oxidized by O
3
produced by an O
3
generator at a temperature of 150°C .
NO
2
in the oxidized flue gas was absorbed by bubbling reactor containing 100ml solution. The initial
and outlet concentrations of NO
2
in the bubbling reactor were quantitatively analyzed by a gas
analyzer.
2.2. Removal efficiency
In this study we use the same amount of ozone as that of NO. We only focused on the removal of
NO
2
. The concentrations of the imported and exported gas of bubbling reactor were measured by
Absorption of NO2 by Sodium Sulfite Solution Adding Ethanol in High Oxygen Concentration
425
bypass lines. NO
2
removal efficiency of the bubbling reactor was calculated using the following
equation:
Where
is the inlet NO
2
concentration of the bubbling reactor and
is the outlet
NO
2
concentration of the bubbling reactor.
3. Results and discussion
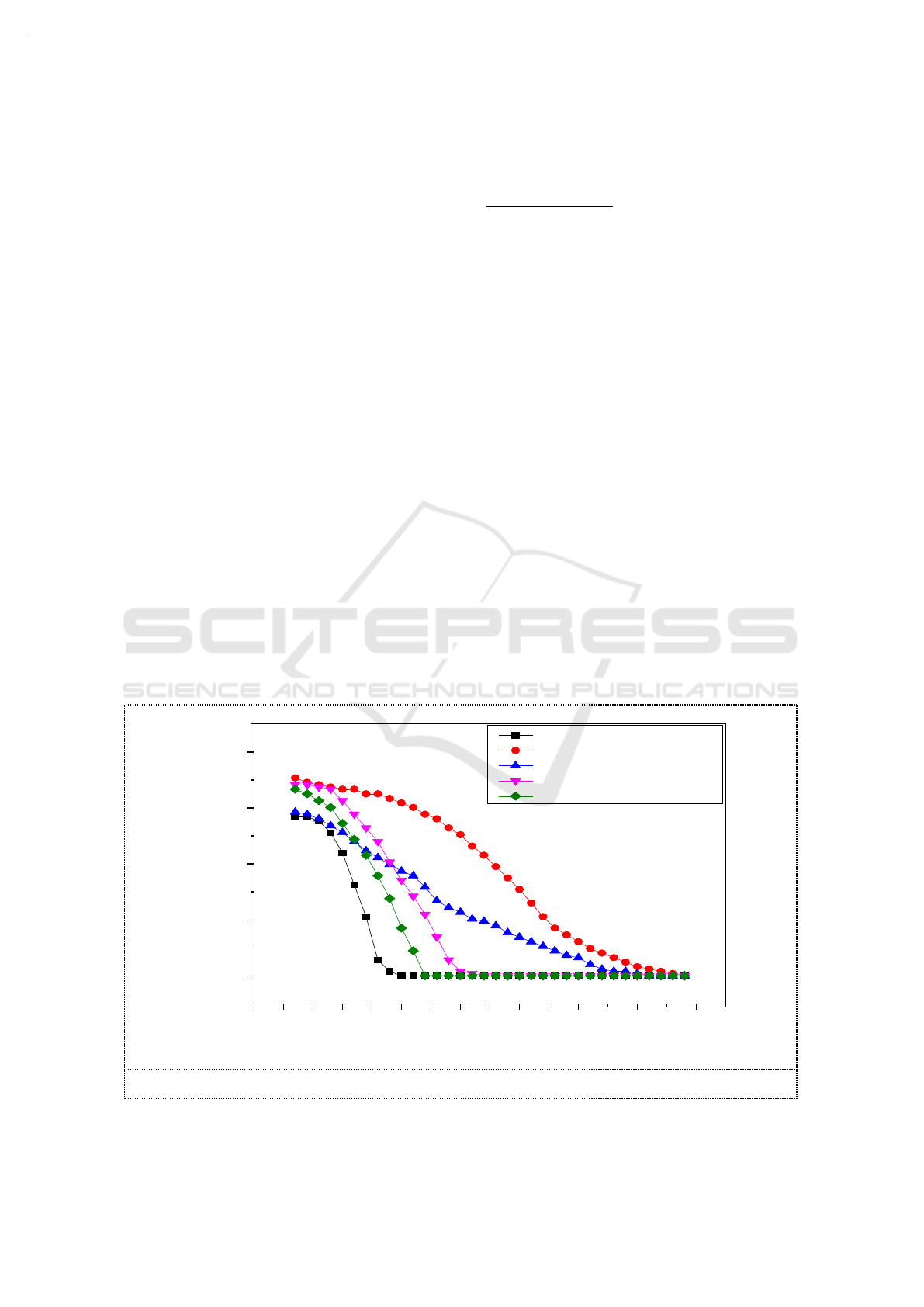
3.1. Selection of additives
According to previous studies, it was found that adding reductive substances to sodium sulfite
solution can inhibit the oxidation of sulfite. [10] Other reports indicated that alcohols are optimal for
the preservation and oxidation inhibition of sodium sulfite solutions. [11] In this study, we used
alcohols as additives in sodium sulfite solution to explore their promotion of the absorption of NO
2
.
While the MR of O
3
and NO were controlled at 1, 0.01mol C
2
H
5
OH ,0.01mol H
2
C
2
O
4
,0.01mol
(CH
2
OH)
2
and 0.01mol (CH
3
)
2
CHOH were added respectively into 100ml of 3.6g/L Na
2
SO
3
solution
whose pH were adjusted to around 9.5 by NaOH. The temperature of the water bath was set to 55°C.
The absorption efficiency of NO
2
with different additives in Na
2
SO
3
solution are shown in Figure
2. The additives increased NO
2
absorptive capacity of sodium sulfite solution by varying degrees.
Compared with oxalic acid, alcohol additive had a synergistic effect on initial NO
2
absorption
efficiency. With the prolongation of reaction time, the synergistic effect of oxalate was prominent.
The synergistic effect of NO
2
absorption was more efficient than that of ethylene glycol or propanol.
NO
2
absorption with the addition of ethanol was much higher than that of the other additives,
regardless of the initial NO
2
absorption rate or the NO
2
absorption capacity of the sodium sulfite
solution.The removal efficiency of NO
2
increased from 60% to 70%. The effective duration increased
from 7min to 32min . As an additive of sodium sulfite solution, ethanol had a great significant
synergistic effect on the absorption of NO
2
with a high oxygen concentration (15%).
0 5 10 15 20 25 30 35
0
20
40
60
80
NO2 removal rate
(%)
time(min)
Na2SO3
Na2SO3+C2H5OH
Na2SO3+H2C2O4
Na2SO3+(CH2OH)2
Na2SO3+(CH3)2CHOH
Figure 2. Absorption efficiency of NO
2
with different additives in Na
2
SO
3
solution.
IWEMSE 2018 - International Workshop on Environmental Management, Science and Engineering
426
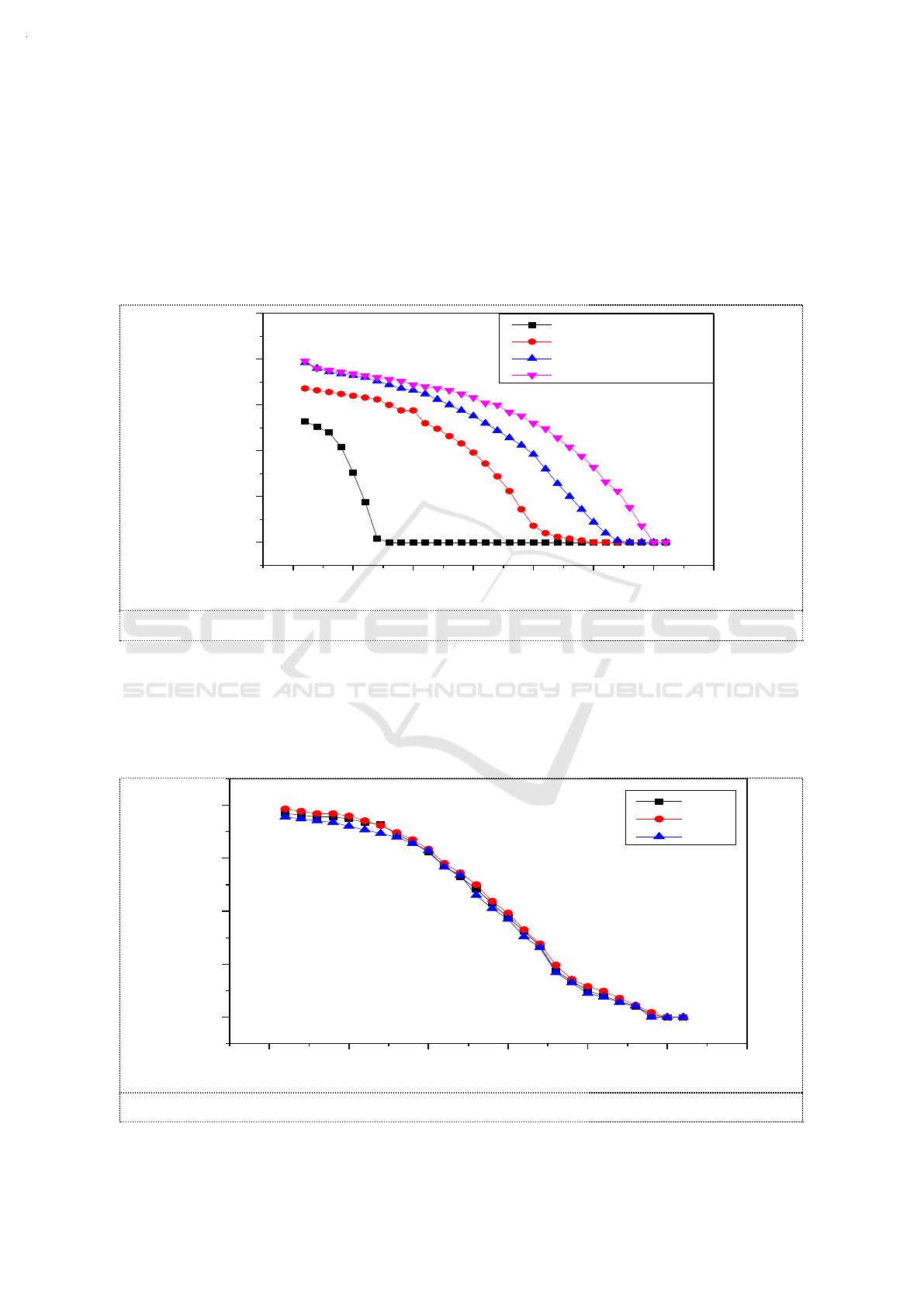
3.2. The effect of the concentration of ethanol on the absorption of NO
2
in Na
2
SO
3
solution
While MR of O
3
and NO were controlled at 1, 0.005mol C
2
H
5
OH,0.01mol C
2
H
5
OH,0.02mol
C
2
H
5
OH were added respectively into 100ml of 3.6g/L Na
2
SO
3
solutions. The effect of the
concentration of ethanol on the absorption of NO
2
in Na
2
SO
3
solution is shown in the figure 3.
The increase of ethanol concentration in the absorption solution enhanced the absorptive capacity
of NO
2
. The removal efficiency of NO
2
increased from 50% to 80% when ethanol concentration
increased from 0 to 0.2mol/L. Meanwhile the effective duration increased from 7min to 32min.
0 5 10 15 20 25 30 35
0
20
40
60
80
100
NO2 removal rate(%)
time(min)
0mol/LC2H5OH
0.05mol/LC2H5OH
0.1mol/LC2H5OH
0.2mol/LC2H5OH
Figure 3. The effect of the concentration of ethanol on the absorption of NO
2
in Na
2
SO
3
solution.
3.3. The effect of temperature on absorption of NO
2
While MR of O
3
and NO was controlled at 1, the water bath was set at35 ℃, 55 ℃and75 ℃. Then,
100ml of 3.6g/L Na
2
SO
3
solution was added to 0.01mol/L C
2
H
5
OH, and the absorption of NO
2
was
investigated. As shown in Figure 4, the reaction temperature of the liquid phase had little effect on
the absorption of NO
2
.
0 5 10 15 20 25 30
0
20
40
60
80
NO2 removal rate(%)
time(min)
35℃
55℃
75℃
Figure 4. The effect of temperature on absorption of NO
2.
Absorption of NO2 by Sodium Sulfite Solution Adding Ethanol in High Oxygen Concentration
427
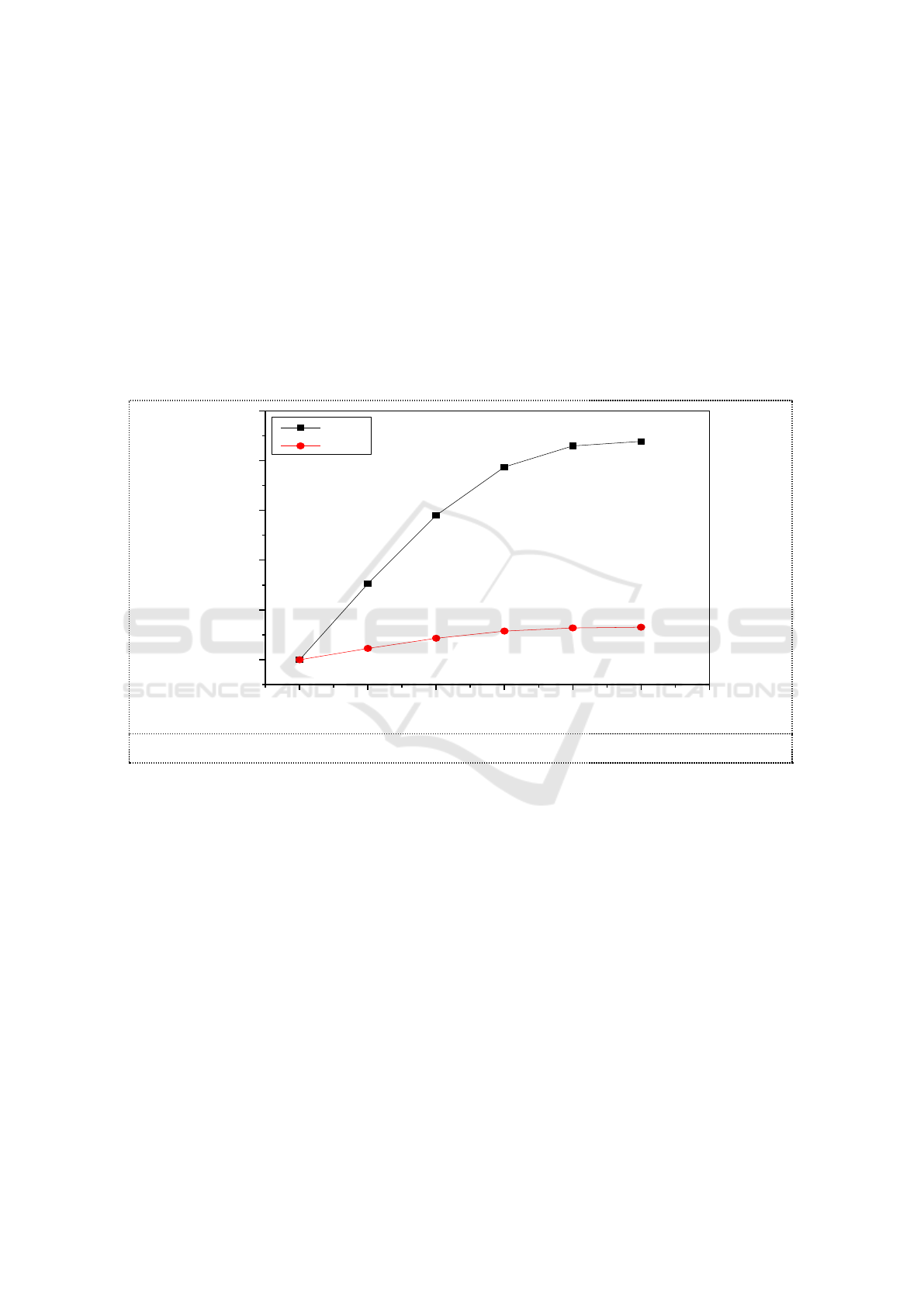
3.4. Ions in the absorption solutions
While MR of O
3
and NO were controlled at 1, 0.01mol C
2
H
5
OH were added into 100ml of 3.6g/L
Na
2
SO
3
solution. The temperature of water bathe was set at 55 ℃. The reaction time was controlled
respectively at 5min, 10min, 15min, 25min. NO
3
-
and NO
2
-
in reaction solutions were measured by
using Ion chromatograph. The concentrations of ions in the absorption solutions are shown in picture
5.
In the absorption of NO
2
, NO
2
-
was the main product of oxidation-reduction reaction in the
solution. The concentration of NO
3
-
in the solution was only 10mg/L. This shows that the direct
product of the reaction between NO
2
and SO
3
2-
in the solution was NO
2
-
. The results were the same as
that of previous studies [12]
in Which Na
2
SO
3
solution absorbed NO
2
without additives. The addition
of ethanol to sodium sulfite did not change the product of its absorption of NO
2
. A small amount of
NO
3
-
in the solution is produced by the hydrolysis of NO
2
.
0 5 10 15 20 25 30
0
20
40
60
80
100
Ion concentration
(mg/L
)
time (min)
NO2-
NO3-
Figure 5. Ions in the absorption liquid.
3.5. The effect of ion enrichment on absorption of NO
2
According to ion detection, it was found that NO
3
-
and NO
2
-
are the products in the process of NO
2
absorption. When these two ions are enriched in solution, they might have an adverse effect on NO
2
absorption. NaNO
3
and NaNO
2
of different quality were added respectively into 100ml of 3.6g/L
Na
2
SO
3
, 0.1mol/L C
2
H
5
OH. Effect of ion enrichment on absorption of NO
2
in absorbent was shown
in the Figure 6 and Figure 7.
The addition of NO
3
-
caused a slight decrease in the initial absorption rate of NO
2
but did not
change the effective time for the solution to absorb NO
2
.The addition of NO
2
-
had a significant
impact on the absorption of NO
2
including initial absorption rate of NO
2
and the effective duration.
These results showed that NO
2
-
was a direct product of the absorption of NO
2
.
IWEMSE 2018 - International Workshop on Environmental Management, Science and Engineering
428
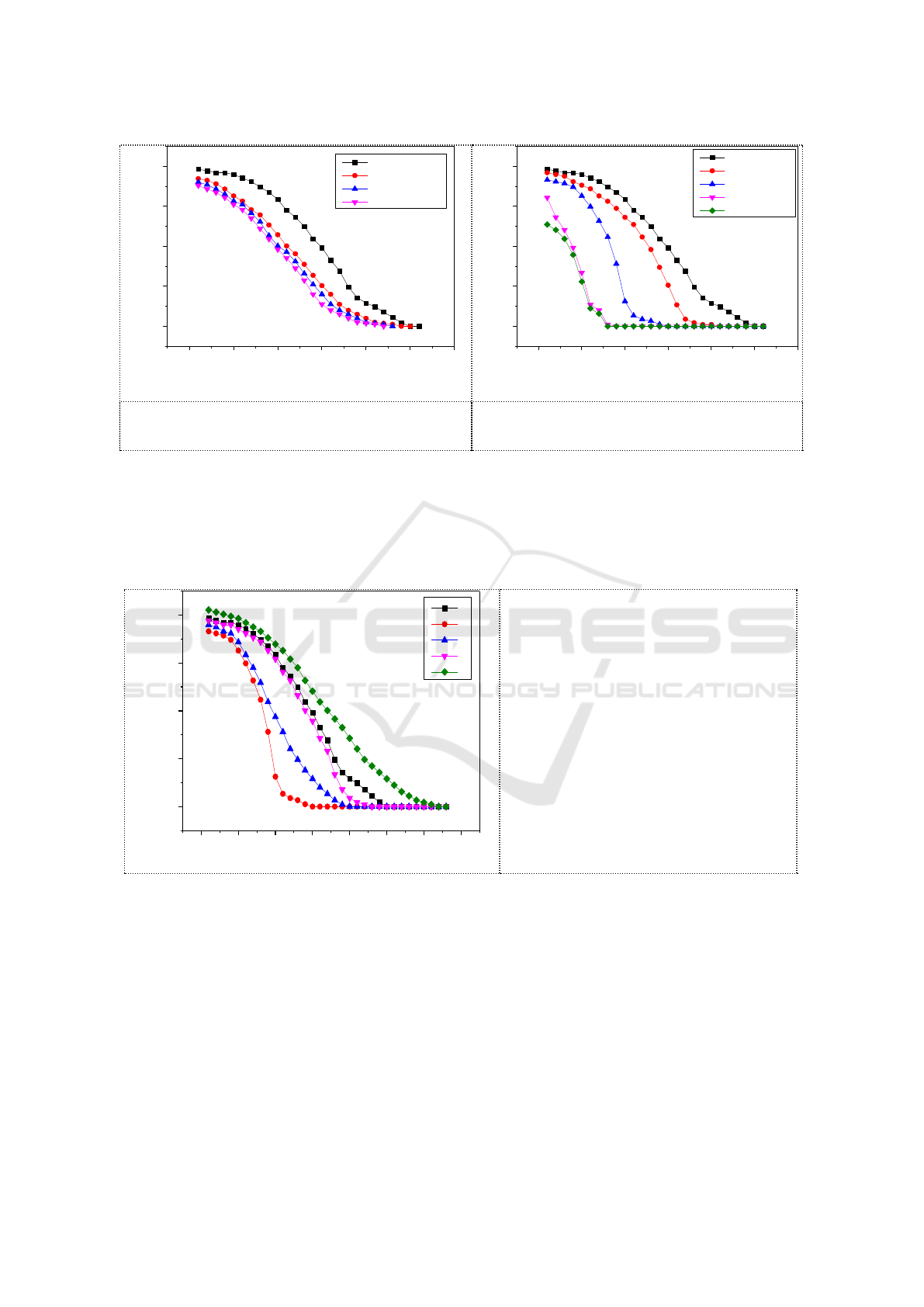
0 5 10 15 20 25 30
0
20
40
60
80
NO2 removal rate(%)
time(min)
0g/LNaNO3
50g/LNaNO3
100g/LNaNO3
200g/LNaNO3
0 5 10 15 20 25 30
0
20
40
60
80
NO2 removal rate(%)
time(min)
0g/LNaNO2
2g/LNaNO2
10g/LNaNO2
20g/LNaNO2
50g/LNaNO2
Figure 6. Effect of NO
3
-
enrichment on NO
2
absorption.
Figure 7. Effect of NO
2
-
enrichment on NO
2
absorption.
Increasing the concentration of ethanol in the solution was found to counteract the effect of ion
enrichment on NO
2
absorption. As shown in Figure 8. NO
2
absorption capacity of 10g/L
NaNO
3
,10g/L NaNO
2
and 3.6g/LNa
2
SO
3
solution added into 0.015mol C
2
H
5
OH reached equal levels
to that of 3.6g/L Na
2
SO
3
and 0.1mol/L C
2
H
5
OH solution. This showed that Increasing the
concentration of ethanol could cancel the effect of ion enrichment on NO
2
absorption
0 5 10 15 20 25 30 35
0
20
40
60
80
NO2 removal rate
(%)
time(min)
a
b
c
d
e
Figure 8. Cancellation of the effect of
ion enrichment on NO
2
absorption.
a.0.1mol/LC
2
H
5
OH
b.10g/LNaNO
3
+10g/LNaNO
2
+0.05mol/
LC
2
H
5
OH
c.10g/LNaNO
3
+10g/LNaNO
2
+0.1mol/L
C
2
H
5
OH
d.10g/LNaNO
3
+10g/LNaNO
2
+0.15mol/
LC
2
H
5
OH
e.10g/LNaNO
3
+10g/LNaNO
2
+0.2mol/L
C
2
H
5
OH
4. Conclusions
This study researched that sodium sulfite solution with added ethanol was an effective absorbent in
high oxygen concentration. The increase in ethanol concentration in absorption solution enhanced its
NO
2
absorptive capacity. In the process of NO
2
absorption, the reaction temperature of the liquid
phase had little effect on the absorption of NO
2
. NO
2
-
was a direct product of the process of NO
2
absorption. The addition of ethanol to sodium sulfite did not change the product of its absorption of
NO
2
. Increasing the concentration of ethanol could cancel the effect of ion enrichment on NO
2
absorption
Absorption of NO2 by Sodium Sulfite Solution Adding Ethanol in High Oxygen Concentration
429

Acknowledgement
The authors gratefully acknowledge the support from the National Key R&D Program of China
(2017YFB0603202), the Key R&D Program of Shandong Province (2016CYJS10B02), and the
Fundamental Research Funds of Shandong University (2017JC012)
References
[1] Zou Z S and Wang C 2007 China J. Iron & Steel 42 8 17
[2] Luo H B 2016 J. Chemical Fertilizer Design 54 2 40
[3] Chen G, Gao J, Gao J and et al 2010 China J. Industrial & Engineering Chemistry Research
49 23
[4] Mok Y S and Lee H J 2006 Korea J. Fuel Processing Technology 87 7 591
[5] Zhuang Z, Sun C, Zhao N and et al 2016 China J. Journal of Chemical Technology &
Biotechnology 91 4 994
[6] Guo S, Lv L, Jia Z and et al 2015 China J. Chemical Industry & Chemical Engineering
Quarterly 21 00 29
[7] Sun C, Zhao N, Wang H and et al 2015 China J. Energy & Fuels 29 5
[8] Chen L, Lin J W and Yang C L 2002 Taiwan J. Environmental Progress & Sustainable
Energy 21 4 225
[9] Tang N, Liu Y, Wang H and et al 2010ChinaJ. Chemical Engineering Journal 160 1 145
[10] Chen L, Lin K F and Yang C L 2011 Taiwan J. Environmental Progress & Sustainable Energy
30 4 632.
[11] Wang L D, Ma Y L, Hao J M and et al 2009 China J. Industrial & Engineering Chemistry
Research 48 9 4307
[12] Takeuchi H and Yamanaka Y 1978 Japan J. Industrial & Engineering Chemistry Process
Design & Development 17 17 389
IWEMSE 2018 - International Workshop on Environmental Management, Science and Engineering
430
