
Production of Rabbit Anti-Excretory/Secretory Product of Fasciola
gigantica Lombok Isolate Antibody
Made Sriasih
1
, Sulaiman Ngongu Depamede
1
, Djoko Kisworo
1
, Galuh Tresnani
2
1
Faculty of Animal Science, Mataram University, Jalan Majapahit No.62 Mataram
2
Faculty of Mathematics and Science, Mataram University, Jalan Majapahit No. 62 Mataram
Keywords: Excretory/Secretory Product, Fasciola gigantica Lombok Isolate, Polyclonal Antibody, ELISA.
Abstract: The major limiting factor in immunodiagnostic development for Fasciolosis detection is the absence of
specific, monoclonal or polyclonal, antibodies against Fasciola antigens specifically Fasciola gigantica,
which is the main species responsible for Fasciolosis in ruminants in Indonesia. The purpose of this study is
to produce polyclonal antibody against excretory/secretory (ES) product of F. gigantica Lombok isolate using
rabbit. Four months old rabbits were immunized subcutaneously with 0.5ml (300μg/ml) of the ES product
emulsified with 0.5ml Freund’s complete adjuvant and then Freund’s incomplete adjuvant. Serum antibodies
were harvested after two-times booster at 1-month interval and determined using enzyme linked-
immunosorbent assay (ELISA) and Western blotting. Immunization results evaluated by ELISA show that
rabbits could be used as a bioreactor to produce ES antibodies. The anti-ES antibody response could be
detected 4 weeks post-immunization, followed by increased humoral response of rabbit after first booster.
The optical density (OD
450nm
) value in the ELISA increased from 0.4 before immunization to 1.184 and 1.392
depending on the type of blocking agent used. The Western blotting results confirm that the ES protein bands
were only recognized by rabbit serum samples post-immunization and thus confirmed the ELISA test result.
1 INTRODUCTION
One of the strategies in early diagnosis of Fasciolosis
caused by Fasciola sp. is the development of
detection methods that can indicate the presence of
disease before it develops into chronic through
immunologic approaches based on antibody detection
or antigen detection. However, the major limiting
factor in the development of immunodiagnostic
methods to detect Fasciolosis in livestock is the
absence of specific, monoclonal or polyclonal,
antibodies against Fasciola sp. antigens. Thus, the
antibody production will be able to overcome the
major inhibiting factor.
Fasciola sp. (liver fluke) that resides inside the
infected host release a significant amount of
excretory/secretory (ES) products (Spithill et al.,
1999), and they play an important role in the
avoidance of parasites from the host immune system
(Morphew et al., 2007). A one-dimension
electrophoresis gel analysis shows that ES products
are composed of proteins of different molecular
weight. Several studies have reported that geographic
variation and different species of fluke will affect the
components of the ES products produced. Sriasih et
al. (2013) stated that one-dimension gel
electrophoresis of the ES products isolated from
Fasciola gigantica that infest Bali cattle in Lombok
island showed protein bands with molecular weight
between 7-25 kDa. The results of the study indicated
that there is a difference in the components of the ES
proteins of F. gigantica Lombok isolate with the
results of previous studies reported by Meshgi et al.
(2008) and Estuningsih et al. (2004).
Studies show that ES products have potential as
an immunoprophylactic agent against Fasciolosis
(Acosta et al., 2008; Jayaraj et al., 2009). Moreover,
ES products are immunogenic so that are potentially
used as vaccine candidates (Ortiz et al., 2000;
Sethadavit et al., 2009), and are used for the diagnosis
of Fasciola-specific antibodies in livestock (Sriasih et
al., 2005; El Ridi et al., 2007; Kooshan et al., 2010).
This indicates that the ES products from the Fasciola
sp. may be potentially used as an antigen to produce
antibodies.
578
Sriasih, M., Depamede, S., Kisworo, D. and Tresnani, G.
Production of Rabbit Anti-Excretory/Secretory Product of Fasciola gigantica Lombok Isolate Antibody.
DOI: 10.5220/0007547405780582
In Proceedings of the 2nd International Conference Postgraduate School (ICPS 2018), pages 578-582
ISBN: 978-989-758-348-3
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

Antibody may be produced by inducing
laboratory animals, such as rats, guinea pigs and
rabbits, with an immunogenic antigen. Immune
response induced by antigen after exposure can then
be measured and determined using serological
testing. Serologic tests developed today can be
divided into two categories; primary binding and
secondary binding tests. The primary binding test is a
test that directly measures the antigenic bonding to
antibodies including fluorescence antibody technique
(FAT), radioimmunoassay (RIA) and Enzyme Linked
Immunoassay (ELISA). The secondary binding test is
a test that measures the results of antigen-antibody
interaction in-vitro which includes agar gel
precipitation est (AGPT), serum agglutination test
and complement fixation test (FAT). However, the
primary binding is more sensitive than the secondary
binding test.
The purpose of this study is to produce polyclonal
antibody against the ES product of F. gigantica
Lombok isolate using rabbit and to determine the
antibody response of rabbit to the ES product using
the ELISA test, and Western blotting. The success in
producing specific antibodies against specific
antigens will increase sensitivity and specificity of
immunodiagnostic test to perform early detection of
Fasciolosis in livestock.
2 MATERIALS AND METHODS
2.1 Research Design
Two local rabbits (4 months old) were placed in a
special cage and fed with pellets, fresh vegetables,
and drinking water (ad-libitum). The rabbits were
then immunized with 0.5ml (300μg / ml) of
emulsified ES product with 0.5ml Freund's complete
adjuvant (FCA). Sera were harvested and tested with
the ELISA before initial immunization and after
booster. The ELISA results were then confirmed by
Western blot analysis.
2.2 Immunization and Serum
Collection
Animal Ethics approval (Protocol No.
235/UN18.8/ETIK/2017) had been obtained for
conducting this study. Immunization was carried out
by subcutaneous injection at multiple sites (no more
than 5) behind the neck and was dispersed under the
skin by gentle rubbing. Four weeks after initial
immunization with the ES product emulsified with
the FCA, the rabbits were then immunized twice
subcutaneously with 0.5ml (300 μg / ml) of the ES
product mixed with 0.5ml Freund's incomplete
adjuvant (FICA) at 1-month interval.
Blood sampling was performed by taking blood
from the auricularis vein of the rabbits using a 3ml
syringe. Blood were placed on a sterile tube and were
then incubated at room temperature for 3 hours. After
incubation, the serum can be obtained by
centrifugation at 5000rpm at 4
o
C for 15 min. The sera
were then aliquoted into several eppendorf tubes and
then stored at -20°C until further assays.
2.3 Measurement of Humoral Immune
Response
Humoral immune response of each rabbit to the ES
product was measured by ELISA technique, and then
confirmed by Western blot. ELISA was performed
according to Sriasih’s procedure (Sriasih et al., 2005).
The ELISA plates (96 wells) were coated with 50 μl
of the ES product and incubated for 1 hour at room
temperature. After incubation, the ES product was
removed and the wells were washed 5 times using
phosphate buffer saline (PBS) containing 0.05%
Tween 20. Blocking agent (100 μl PBS containing
bovine serum albumin (BSA) or skim milk) or were
added into each well and were then incubated for 1
hour at room temperature. Following incubation, the
plate was washed again (5 times washing cycling).
Fifty microliters serum that had been diluted a
hundred times were added into each well and then
incubated for 1 hour at room temperature. After 5
times washing, 50μl anti-rabbit IgG horse-radish
peroxidase conjugated were added and then incubated
for 1 hour. One hundred microliters of substrate
(ABTS in 100ml of citrate buffer) were added into the
wells and incubated for 15 min room temperature.
Optical density (OD) was then measured at 405nm
wavelength using an ELISA reader machine.
2.4 Western Blotting Analysis
After electrophoresis, gels were equilibrated in
transfer buffer for at least 10 min. Polyvinylidine
difluoride (PVDF) membrane was pre-incubated in
100% methanol for 1 min then rinsed with several
changes of water. After rinsing, the membrane and
filter papers were also allowed to equilibrate for at
least 15 min in transfer buffer. The transfer of
proteins from the gel to the membrane was carried out
at a constant voltage of 15 Volts for 35-40 minutes
using a Trans-Blot® Semi-Dry (SD) electrophoretic
transfer cell as per the manufacturer’s instructions.
Production of Rabbit Anti-Excretory/Secretory Product of Fasciola gigantica Lombok Isolate Antibody
579
For immunostaining, the membranes were
washed multiple times in distilled water and
immediately blocked with blocking buffer (Tris
buffer saline pH 7.4 [20 mM Tris-HCl, 100 mM
NaCl] containing 5% [w/v] skim milk and 0.1% [v/v]
Tween-20) at room temperature for 1 hour. After
washing twice in washing buffer (Tris buffer saline
pH 7.4 [20 mM Tris-HCl, 100 mM NaCl] containing
0.1% [v/v] Tween-20), the membrane was cut into
strips and further incubation of each strip was carried
out in individual reservoirs. Each strip was incubated
with 10 ml of diluted rabbit sera (1:500) for 1 hour at
room temperature. Each strip was then washed for
five 5-10 min cycles with washing buffer. After
washing, diluted anti-rabbit IgG HRP (1:8000) was
applied and incubated for 1 hour at room temperature.
Strips were then washed as previously described.
Immunodetection was developed by addition of TMB
substrate.
2.5 Data Analysis
Humoral immune responses of each rabbit were
recorded and analyzed using a simple statistical
calculation (Mean ± Standard Deviation).
3 RESULTS AND DISCUSSION
The ES product used for rabbit immunization in this
study was derived from the Lombok isolate of
F.gigantica (Sriasih et al., 2013). The ES products
were injected into the rabbits as a bioreactor to
produce the ES antibodies because they have a distant
genetic relationship with cattle, are easy to handle and
easy to maintain. Rabbits are also laboratory animals
that are widely used in various studies for the
production of antibodies, tumorigenesis, nutrition,
genetics, radiation research and anaphylactic
research. The immune system of the rabbit will
recognize and react to the antigen. The lymphocyte
cells exposed as part of the body's defense system
then will multiply and develop into plasma cells that
produce antibodies. The antibodies formed are
polyclonal antibodies with varied composition in
serum, either as a result of repeated immunization, or
due to variations that occur during an immune
reaction (Tizard, 2004).
The ELISA test results of humoral rabbit response
before immunization (baseline), 4 weeks after the
initial immunization (FCA), 4 weeks after the second
immunization (FICA1) and 2 weeks after the third
immunization (FICA2) using two blocking agents,
BSA and non-fat dry milk are presented in Table 1.
Table 1: The humoral response of the rabbits to the ES
antigen on ELISA test.
Data on Table 1 depict that only rabbit #2 showed
a good immune response. The baseline accounted for
an optical density value of 0.459 in the ELISA with
BSA blocking and 0.548 with non-fat dry milk
blocking. After immunization with the emulsified ES
antigen with FCA, the optical density value increased
to 1.184 (BSA) and 1.392 (non-fat dry milk). This
may occur as a result of activation of the B cells
memory whose work is stimulated by T cells to
produce antibodies in large quantities (Goldsby et al.,
2000; Abbas et al., 2007).
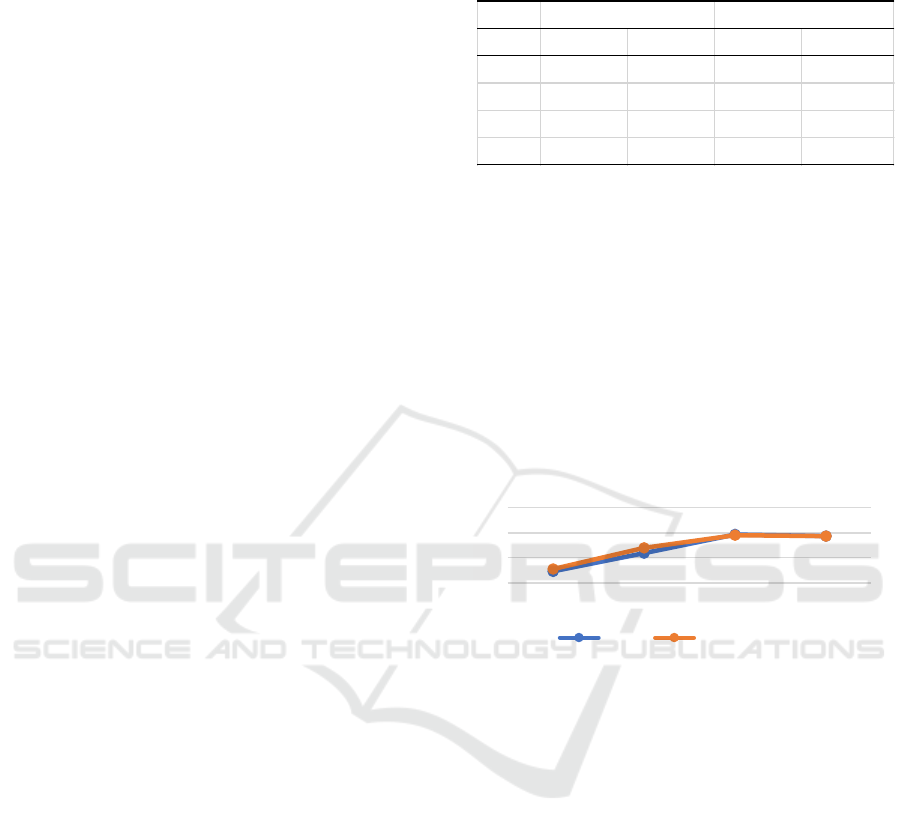
Figure 1: Polyclonal immune response of rabbit#2 anti ES
antibody blocked with different blocking agents.
The use of different blocking agents in this study
(Figure 1) did not have a significant effect on the
optical density values in the ELISA test. Both BSA
and non-fat dry milk are regularly used blocking
agents in the ELISA test. Milk contains a number of
different proteins, and one of them is casein
phosphoprotein. This phosphoprotein can cause a
high background value due to the non-specific
reaction to the phospho structure.
The Western blotting result (Figure 2) also
corroborated the ELISA assay results and showed that
the ES protein bands were only recognized by the
rabbit serum (rabbit #2) after immunization. Cruse
and Lewis (2002) suggest that the addition of
adjuvants to the injected isolate serves to enhance the
immunogenicity of the isolate. The presence of
Mycobacterium sp in Freund’s complete adjuvant in
early immunization will stimulate B cells and T cells
to produce an immune response. The primary
immune response of B cells is activated to proliferate
Rabbit #1 Rabbit #2 Rabbit #1 Rabbit #2
Baseline 1.866±0.002 0.459±0.013 1.628±0.01 0.548±0.007
FCA 2.06±0.012 1.184±0.011 1.729±0.008 1.392±0.001
FICA1 2.135±0.005 1.928±0.007 1.772±0.003 1.901±0.002
FICA2 2.165±0.006 1.867±0.002 1.783±0.002 1.864±0.002
BSA Blocking
Non-fat dry milk blocking
0
1
2
3
Baseline FCA FICA1 FICA2
Humoral immune response of rabbit# 2
BSA Non-fat dry milk
ICPS 2018 - 2nd International Conference Postgraduate School
580

and differentiate within antibody secretion cells and
memory cells. Some antibody cells migrate and
survive in the bone marrow for long periods. The
second immunization (booster 1) and the third
immunization (booster 2) with Freund’s incomplete
adjuvant will produce secondary immune response
with higher concentration than the first immunization.
Figure 2: Western blot analysis of rabbit anti-ES polyclonal
antibodies. MW = standard protein marker; 1 = rabbit pre-
immune sera; 2 = rabbit sera collected after second booster.
The formation of rabbit antibodies is influenced
by the antigenicity of the injected ES proteins. The
principal feature of a substance or compound is
determined by physicochemical limitation and degree
of foreignness (Tizard, 2004). The physicochemical
limitation of a substance or compound is that the size
of the antigen molecule must be large, rigid and has a
complex chemical structure (Kuby, 2007). The
chemical structure of the ES proteins derived from the
F. gigantica Lombok isolate that are large and
complex will produce faster antibodies.
The results of this study indicate that rabbit can be
used as a manufacturer to produce the ES antibodies.
Antibodies formed 4 weeks post immunization,
followed by increased humoral response of the rabbits
after booster 1. Setyaningsih (2004) in her study using
rabbits to produce polyclonal antibody against ES
proteins isolated from F.gigantica buffalo isolate and
goat isolate reported that antibodies anti-ES of F.
gigantica goat isolate are formed faster than that of
buffalo isolate. Antibodies anti-ES of F. gigantica
goat isolate were formed at 4 weeks while antibodies
anti-ES of F. gigantica buffalo isolate formed at 12
weeks. The time difference to elicit antibody-forming
responses to the host may vary and is dependent on
the immunogenicity, the form and stability of
stimulants, animal species, injection routes, and the
sensitivity of tests used to detect antibodies formation
(Tizard, 2004). The host's response to the
immunogens given is not only determined by the
immunogenic physical properties but is also
determined by host-related factors such as genetics,
age, nutritional status and secondary effects derived
from a disease.
4 CONCLUSIONS
Anti-ES polyclonal antibody specific to the ES
antigens of F. gigantica Lombok isolate has been
successfully produced in rabbit. The availability of
the antibody will certainly facilitate the development
of a better immunodiagnostic tests for controlling
Fasciolosis.
ACKNOWLEDGEMENT
This study was funded by the Ministry of Research,
Technology and Higher Education. The authors also
would like to thank Postgraduate study program of
Mataram University for travel grant given.
REFERENCES
Abbas, A. K., A. H. Lichtman, S. Pillai. 2007. Cellular and
Mollecular Immunology. 6th Ed. Philadelphia:
Elsevier Inc.
Acosta, D., M. Cancela, L. Piacenza, L. Roche, C.
Carmona, J.F. Tort. 2008. Fasciola hepatica leucine
aminopeptidase, a promising candidate for vaccination
against ruminant Fasciolosis. Mol. Biochem.
Parasitol. 158: 52-64.
El Ridi, R., M. Salah, A. Wagih, H. William, H. Tallima,
M.H. El Shafie, T. Abdel Khalek, A. El Amir, F.F. Abo
Ammou, H. Motawi. 2007. Fasciola gigantica
excretory-secretory products for immunodiagnosis and
prevention of sheep Fasciolosis. Vet. Parasitol. 149:
219-228.
Estuningsih, S.E., S. Widjajanti dan G. Adiwinata. 2004.
Perbandingan antara uji ELISA-Antibodi dan
pemeriksaan telur cacing untuk mendeteksi infeksi
Fasciola gigantica pada sapi. JITV 9: 55-60.
Goldsby, R. A., T. J. Kindt, B. A. Osborne. 2000.
Immunology. 4th Ed. New York: W. H. Freeman & Co.
Jayaraj, R., D. Piedrafita, K. Dynon, R. Grams, T.W.
Spithill, P.M. Smooker. 2009. Vaccination against
Fasciolosis by a multivalent vaccine of stage-specific
antigens. Vet. Parasitol. 160: 230-236.
Kooshan, M., G.R. Hashemi and A. Naghibi. 2010. Use of
somatic and excretory-secretory antigens of Fasciola
hepatica in diagnosis of sheep by ELISA. American-
Eurasian J. Agric. & Environ, Sci. 7 (2): 170-175.
Kuby, J. 2007. Immunlogy 6th Ed. New York: W. H.
Freeman Company.
Production of Rabbit Anti-Excretory/Secretory Product of Fasciola gigantica Lombok Isolate Antibody
581

Meshgi, B., A. Eslami, F. Hemmatzadeh. 2008.
Determination of somatic and excretory-secretory
antigens of Fasciola hepatica and Fasciola gigantica
using SDS-PAGE. Iranian J. Vet. Res. 9 (1): 77-80.
Morphew, R.M., H.A. Wright, E.J. LaCourse, D.J. Woods,
P.M. Brophy. 2007. Comparative proteomics of
excretory-secretory proteins released by the liver fluke
Fasciola hepatica in sheep host bile and during in vitro
culture ex host. Mol. And Cell. Proteom. 6 (6): 963-971.
Ortiz, P.L., J.R. Claxton, M.J. Clarkson, J. McGarry, D.J.
Williams. 2000. The specificity of antibody responses
in cattle naturally exposed to Fasciola hepatica. Vet.
Parasitol. 121-134.
Sethadavit, M., K. Meemon, A. Jardim, T.W. Spithill, P.
Sobhon. 2009. Identification, expression and
immunolocalization of cathepsin B3, a stage-specific
antigen expressed by juvenile Fasciola gigantica. Acta
Trop. 112: 164-173.
Setyaningsih, R. 2004. Produksi antibodi poliklonal anti
ekskretori sekretori (ES) Fascciola gigantica pada
kelinci. Skripsi. Fakultas Kedokteran Hewan, Institut
Pertanian Bogor, Bogor.
Spithill, T.W., P.M. Smooker, D.B. Copeman. 1999.
Fasciola gigantica: epidemiology, control,
immunology and molecular biology. In: Dalton, J.P.
(Ed), Fasciolosis. CAB International, Wallingford, pp.
465-525.
Sriasih, M., E. Yulianti, Khalid. 2005. Penggunaan hasil
ekskresi/sekresi Fasciola gigantica sebagai antigen
untuk deteksi Fasciolosis pada sapi. Laporan
Penelitian. Hibah UPT MIPA-Unram.
Sriasih, M., D. N. Sulaiman dan M. Ali. 2013. Karakterisasi
protein antigenik cairan ekskretori/sekretori cacing F.
gigantica isolat lokal dengan teknik Western blotting.
Laporan Penelitian, Universitas Mataram.
Tizard, I. R. 2004. An Introduction to Veterinary
Immunology. 7th Ed. Elsevier: Philadelphia.
ICPS 2018 - 2nd International Conference Postgraduate School
582
