
The Effect of Tadoples (Rana catesbeiana) Serum on Total and
Differential Leukocyte in Rats (Rattus norvegicus): That Have Been
Induced With Dimetylbenz-α-anthracene
as Animal MODEL of Skin Cancer
Nur Prabowo Dwi Cahyo
1
,Prima Ayu Wibawati
2
,
Hardany Primarizky
3,
Ragil Angga Prastiya
4
,
Muhammad Thohawi Elziyad Purnama
5
and Ratna Damayanti
6
1a
Student Faculty of Veterinary Medicine, Universitas Airlangga
,
b
Post Graduate School Universitas Airlangga
2
Department of Veterinary Public Health,
3
Department of Veterinary Clinic
4
Department of Veterinary Reproduction,
5
Department of Veterinary Anatomy,
6a
Departement Basic Veterinary Medicine Faculty of Veterinary Medicine, Universitas Airlangga
6b
Post Graduate School Universitas Airlangga
Keywords: Total leukocyte, differential leukocyte , Rana catesbeiana, serum, skin cancer.
Abstract: This study aimed to determine the effect of tadpoles (Rana catesbeiana) serum on total and differential
leukocyte in animal model of white rat (Rattus norvegicus) that has been induced with skin cancer by
Dimethylbenz-α-anthracene (DMBA). Male rats were induced by DMBA 20 mg/rat twice a week for 18
days to induce skin cancer. Tadpole’s serum was injected intracutaneously after cancer had been known.
Negative control (K-) was not induced with DMBA and tadpole’s serum, while positive control grup (K+)
was induced to DMBA. The treatment groups P1, P2, and P3 were induced with DMBA and injected
tadpole’s serum 100%; 75%; 25%/rat/day. This study used a Completely Randomized Design (CRD). Data
were analyzed with ANOVA and continued by Duncan multiple test. The results obtained average number ±
SD of total leukocyte K-, K+, P1, P2, and P3 were 12000.00±3814.88,3975.00±2451.36,8650.00± 5470.83,
6390.50 ± 3007.18 and 5590.00 ± 1292.18 respectively. There are significant differences regarding an
increase in number of total leukocyte on treatment, but there is not real difference between K+, P1, P2 and
P3. The results shsowed average number of limphocyte and monocyte are not significant but it is signiffant
in granulocyte. Based on the results, it can be concluded that tadpoles serum is effective to increase number
of total leukocyte and differential leukocyte (especially in granulocyte) in animal model of rats induced skin
cancer.
1 INTRODUCTION
The prevalence of cancer in the population of all
ages in Indonesia in 2003 reached 0.14 percent of
the total population or 347,792 people (Ministry of
Health, 2015). According to Indonesian Nutrition
Network (2005), in Indonesia, cancer patients
reached 6% of the population and deaths from this
malignancy ranked second after death from
infection. Based on a research conducted by
Dhaygude (2006) in Mumbai, India during the
period from January 2001 to December 2005, from
124 dogs who were autopsied or biopsy, the most
recorded skin tumor was found in 74 (59.67%)
followed by mammary gland tumor as many as 43
556
Cahyo, N., Wibawati, P., Primarizky, H., Prastiya, R., Purnama, M. and Damayanti, R.
The Effect of Tadoples (Rana catesbeiana) Serum on Total and Differential Leukocyte in Rats (Rattus norvegicus) That Have Been Induced With Dimetylbenz-Î
´
s-anthracene as Animal MODEL
of Skin Cancer.
DOI: 10.5220/0007546905560560
In Proceedings of the 2nd International Conference Postgraduate School (ICPS 2018), pages 556-560
ISBN: 978-989-758-348-3
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

tails (34,67%) and subsequently transmissible
granuloma in genitals as many as 3 tails and ovarian
and testicular tumor were 2 each (1.6%).
According to Mondou and Kaltenbach (1979),
the tadpoles serum (Rana catesbeiana) contains
thyroxine hormones that can enhance cellular
immune responses. Thyroxine content in tadpole
serum is 9.4 ng / ml, while thyroxine needed as
therapy to increase immune is 0.01 - 0.1 microgram /
ml. Leukocytes have a very important role in the
body's defense, so the examination of the number of
leukocytes is to support the diagnosis of disease. The
body has a special system to remove a variety of
infectious and toxic materials, one of which is
leukocytes or white blood cells. Blood test results
can be used as a good parameter and can generally
describe the condition of the body such as the
examination of the total number of leukocytes
(Guyton and Hall, 2011).
There has been no research or data on the total
type of white rat (Rattus norvegicus) leukocytes
induced in DMBA and given serum tadpoles (Rana
catesbeiana). Based on this, a research needs to be
done to find out the effect of tadpoles serum (Rana
catesbeiana) on total white rat leukocytes (Rattus
norvegicus) which suffer from cancer induced
DMBA.
2 MATERIALS AND METHODS
This study used 20 male white rats (Rattus
norvegicus) which weighed around 150-250 grams.
The experimental animals were then divided into 2
control groups (negative and positive) and 3
treatment groups.
The tools used in this research are weight
weighing rats, rat cage, drink container, food
container, litter or cage from wood powder, 1 ml
disposable syringe with tuberculin needle, 100ml
beaker glass, 100 ml measuring cup, analytical
scales, filter paper, and camera. The ingredients for
the cancer-trigger used in this study are DMBA
(7,12-Dimethylbenz-α-anthracene) dissolved in corn
oil. DMBA injection (7,12-Dimethylbenz - α-
anthracene) was done using 1 ml disposable syringe
with tuberculin needle and sterile cotton with 70%
alcohol.
Tadpoles blood-collecting (Rana
catesbeiana) was done using syringe 1 ml disposable
with tuberculin needle. Blood was collected using an
EDTA tube without anticoagulation. The tube was
covered with aluminum foil and centrifuged. Serum
dilution was done using PZ or NaCL physiological
0.9% then injected in white rat (Rattus norvegicus)
using 1 ml disposable syringe with tuberculin
needle.
Dilution of DMBA (7,12-Dimethylbenz-α-
anthracene) was performed before inducing cancer.
Dilution was done using corn oil. Corn oil served as
a solvent of DMBA (7,12-Dimethylbenz-α-
anthrancene). Dosage for DMBA induction (7,12-
Dimethylbenz-α-anthrace) as a trigger for raising
cancer cells was 20 mg / kg BW (Cabecas et al,
2014). DMBA induction (7,12-Dimethylbenz-α-
anthracene) to induce cancer cells was injected
subcutaneously using a 26G size needle. DMBA
powder (7,12-Dimethylbenz-α-anthrance) was
dissolved in advance with corn oil in order to
facilitate the induction process. Comparison of
DMBA powder (7,12-Dimethylbenz-α-anthrance)
with corn oil is 1 ml of corn oil containing 20 mg
DMBA (7.12-Dimethylbenz-α-anthrance). Induction
was done for 14 days with duration of twice a week.
Subcutaneous induction was performed
subcutaneously on the nape of white rats (Rattus
norvegicus). Cancer observations were performed
after the first injection of DMBA (7,12-
Dimethylbenz-α-anthrance) by palpation of the
injection or nape and skin portions of other white
mice (Rattus norvegicus). Cancer observation was
also carried out by the measurement of diameter and
number of nodules that arised. The expected nodule
is a cancer nodule, not an abscess nodule. Palpation
and measurements were made daily.
The negative control group was not induced with
DMBA, whereas the positive control group was
induced with 20 mg/kg BW DMBA. All treatment
groups were induced by 20 mg/kg BW DMBA. The
treatment stage after 14 days was induced with
DMBA and after the appearance of skin nodules, the
white rats treated group were injected with 1.06 ml
tadpoles serum (Mondou dan Kaltenbach, 1979), in
P1(100%), P2 (75%) and P3 (25%). Injecting
tadpoles serum was done once a day for seven days.
Blood sampling was performed through the heart
(Cardiac puncture) using a 2 ml disposable syringe
in rats on the 44
th
day. Blood was then
accommodated in an EDTA tube as to not affect the
size and shape of the erythrocytes or the shape of the
leukocytes (Bijanti et al., 2010). Blood examination
was done using Hematology Analyzer HORIBA
ABX MICROS 60 instrument and then connected
with computer. Blood was homogenized first using
Roller-Mixer for 1-2 minutes before checking using
Hematology Analyzer.
The Effect of Tadoples (Rana catesbeiana) Serum on Total and Differential Leukocyte in Rats (Rattus norvegicus) That Have Been Induced
With Dimetylbenz-Î
´
s-anthracene as Animal MODEL of Skin Cancer
557
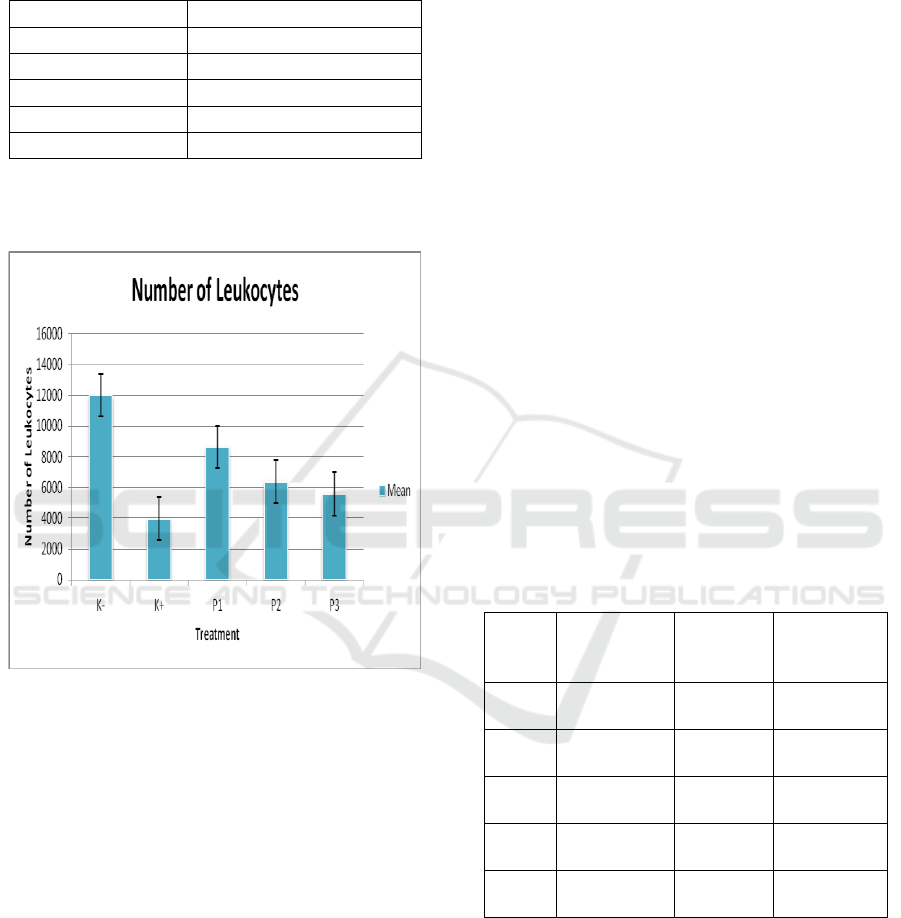
3 RESULT AND DISCUSSION
Table 1 : Mean of Total Number of Leukocytes in White
Rat due to Tadpole Serum (Rana catesbeiana) influence.
Treatment
Mean ± SD
K-
12000,00
a
± 3814,88
K+
3975,00
b
± 2451,36
P1
8650,00
ab
± 5470,83
P2
6390,50
b
± 3007,18
P3
5590,00
b
± 1292,18
Description : Different Superscript in the same
Coloumn shows significant differences (p<0.05)
Figure 1: Diagram of White Rat Leukocyte Due to Giving
Tadpole Serum (Rana catesbeiana).
The K+ group (DMBA 20 mg / kg BB + solvent
corn oil) showed the lowest number of 3,975,00
b
±
2,451,36 compared with the negative group and all
treatment groups, this proves that DMBA can
suppress the activity of bone marrow and splenosit
so that it can reduce the number of leukocytes
especially neutrophils, monocytes and lymphocytes
(Akrom et al., 2013)
In P1, it showed the highest increase of total
leukocyte number which is 8650,00
ab
± 5470,83,
then followed by P2 that is 6,390,50
b
± 3,007,18,
and P3 that is 5,590,00
b
± 1,292,18. The increase in
total leukocytes in the treatment group 1 to treatment
3 showed that the addition of young tailed frog
serum increased the number of cancer-induced white
rat leukocytes (Rattus norvegicus) using DMBA
(Dimethylbenz-α-anthracene). An increase in the
number of leukocytes is estimated as a result from
an increase in the number of natural killer cells.
Tadpoles serum acts as an immunomodulator that
stimulates the immune system, such as improving
macrophage activity, increasing antibodies and
activating natural killer cells. The increase is also
associated with the entry of foreign bodies in the
body and the response of leukocytes as a mean of
body defense (Gigena et al., 2017).
The treatment group showed that higher doses of
tadpoles serum in DMBA-induced rats would
increase the number of leukocytes in the blood. This
result is consistent with the Akrom and Ermawati
(2009) study stated that by administering black
cumin as immunopreventive at higher doses can
increase the number of leukocytes in DMBA-
induced Sprague Dawley rats. The higher the dose of
the tadpoles serum, the higher the number of
leukocytes. It can be said that higher serum doses
can inhibit the development of cancer so that the
number of leukocytes increases. This illustrates the
correlation between the serum content of tadpoles,
thyroxine hormones, and increased immune system
in animals. With the higher doses, the thyroxine
hormone content in tadpole serum is higher, so the
ability to increase the immune system is getting
bigger.
Table 2 : Average and Standard Deviation of White Rat
Leucocyte Types Due to Tadpole Serum (Rana
catesbeiana) Influence.
Treat
ment
Lymphocyt
es (/ mm
3
)
(X ± SD)
Monocyt
e (/ mm
3
)
(X ± SD)
Granulocyt
es (/ mm
3
)
(X ± SD)
K-
9900,00
a
±
3402,94
600,00
a
±
182,57
1500,00
a
±
496,65
K+
3275,00
a
±
2087,06
250,00
a
±
191,48
450,00
b
±
208,16
P1
4575,00
a
±
4601,72
1050,00
a
± 858,29
1225,00
b
±
917,87
P2
5058,50
a
±
2213,10
793,00
a
±
465,25
677,50
b
±
384,74
P3
4549,00
a
±
1298,10
684,00
a
±
138,41
495,50
b
±
189,24
Description: Different superscripts in the same
column show significant differences (p <0.05)
According to Kusumawati (2004), the normal
monocyte value of rats is 0.00 - 0.10 (x 10
3
/ mm
3
).
It can be seen from the average number of
monocytes that positive control group has the lowest
average monocyte count of all treatments. This is
because the positive control of rats were given
cancer by DMBA-induced without any serum
ICPS 2018 - 2nd International Conference Postgraduate School
558

treatment, so that DMBA suppresses bone marrow
activity resulting in decreased monocyte count
(Akrom et al., 2013).
Granulocytes are white blood cells characterized
by granules in the cytoplasm. Granulocytes such as
neutrophils, eosinophils, and basophils are very few
in normal circumstances, but when there is an
antigen the amount will increase (Fitria and Sarto,
2014). In this study, the average number of
granulocytes of the lowest rat in the K+ group was
450,00
b
± 208.16 compared with the K- group and
the all-treatment group. This study shows that
DMBA induction in rats can decrease the average
number of animal granulocytes. In the K- group with
the K+, P1, P2, and P3 groups, there was a marked
difference (p <0.05). The P1, P2, and P3 groups
showed an increase in the number of granulocytes
compared to the K- groups, although the statistics
showed that the increase was not significantly
different (p> 0.05).
4 CONCLUSION
The conclusions of this study are:
1. There was an effect of tadpole serum (Rana
catesbeiana) on the total leucocyte increase of
white rats (Rattus norvegicus) with DMBA-
induced (Dimetylbenz-α-anthracene).
2. There was an effect of tadpole serum (Rana
catesbeiana) on the increase of white rat type
leukocytes (Rattus norvegicus) with DMBA-
induced (Dimetylbenz-α-anthracene).
REFERENCES
Akrom, dan M.I. Ermawati. 2009. Gambaran Jumlah
dan Hitung Jenis Leukosit Serta Waktu
Jendal Darah Pada Tikus Betina yang
Diinduksi 7,12-Dimetilbenz(α)antrasen
(DMBA) Setelah Pemberian Ektrak Etanol
Biji Jinten Hitam (Nigella sativa L). The
Journal of Indonesian Medicinal Plant. Vol
2(2) : 69-78.
Akrom, Mustofa, Marstyawan, Mubarik. 2013.
Chemopreventive and Antihematotoxicity
Effect of Black Cumine Seed Oil. Media
Farmasi. Vol 10 (2) : 56-70.
Alamino, V. A., Mscanfroni, I. D., Montesinos, M.
M., Gigena, N., Donadio, A. C., Blidner, A.
G., Milotich, S. I., Cheng, S. Y., Masini-
Repiso, A. M., Rabinovich, G. A. 2015.
Antitumor Responses Stimulated by
Dendritic Cells are Improved by
Triiodothyronine Binding to The Thyroid
Hormone Receptor Beta. Cancer Res. 75:
1265-74.
Baratawidjaja, K.G. 2004. Imunologi Dasar.
Fakultas Kedokteran Universitas Indonesia.
Jakarta.
Bijanti, R., M. G. A. Yuliani., R.S Wahjuni., dan
R.B Utomo. 2010. Patologi Klinik
Veteriner (Edisi Pertama). Airlangga
University Press (AUP). Surabaya. Hal. 20-
28.
Cabeças, J. R., Costa, E., Alves, G., Jesus, P. dan
Cabrita, A., 2014. Oral Cavity Iorphometric
Evaluation in 7, 12 Dimethylantracene
Administration (1139.11). The FASEB
Journal. 28 (1 Supplement): 1139-11.
Dhaygude, V. 2006. The Study of Canine Mammary
Tumors with Special Reference to
Mutations in p53 Tumor Suppressor Gene
by PCR-SSCP. Masters thesis, College of
Veterinary Science and Animal Husbandry,
Anand Agricultural University, Anand,
Gujurat, India.
Fitria, L. Dan Sarto, M. 2014. Profil Hematologi
Tikus (Rattus norvegicus Berkenhout,
1769) Galur Wistar Jantan dan Betina
Umur 4, 6, dan 8 minggu. Biogenesis 2(2):
94-100. ISSN 2302-1616.
Gigena, N., V.A. Alamino, M. del Mar Montesinos,
M. Nazar, R.A. Louzada, S.M. Wajner,
A.L. Maia, A.M. Masini-Repiso, D.P.
Carvalho, G.A. Cremaschi, dan C.G.
Pellizas. 2017. Dissecting thyroid hormone
transport and metabolism in dendritic
cells. Journal of Endocrinology. 232(2):
337-350.
Girindra, A. 1988. Biokimia Patologi Hewan.
Bogor: PAU IPB.
Guyton, A. C. and Hall, J. E. 2011. Textbook of
Medical .12th ed. Philadelphia, Saunders
Elsevier. pp :423-431
The Effect of Tadoples (Rana catesbeiana) Serum on Total and Differential Leukocyte in Rats (Rattus norvegicus) That Have Been Induced
With Dimetylbenz-Î
´
s-anthracene as Animal MODEL of Skin Cancer
559

Kusumawati, D. 2004. Bersahabat dengan Hewan
Coba. Gajah Mada University Press.
Yogyakarta. p. 9
Mondou,PM and Kaltenbach, JM.1979. Thyroxine
concentrations in blood serum and
pericardial fluid of metamorphosing
tadpoles and of adult frogs. Gen. Comp
Endocrinol,39930 ; 343-9
ICPS 2018 - 2nd International Conference Postgraduate School
560
