
Signal Tranducers and Activators Transcription (STAT) 5b Protein
as A Candidate of Growth Promoter in Broiler Chicken
Anwa Ma’ruf
1, 2
, Ratna Damayanti
2
and Nove Hidajati
2
1
Postgraduate School, Universitas Airlangga, Surabaya, Indonesia
2
Department of Basic Veterinary Medicine, Faculty of Veterinary Medicine, Universitas Airlangga Surabaya, Indonesia
Keywords: STAT 5b, amino acid, growth, broiler
Abstract: The purpose of this study is to determine the molecular weight of the protein STAT-5b that exists in liver
tissue as a basis to determine the amino acid composition of broiler STAT protein phosphatase that
experiences growth due to increased growth hormone (GH). Broiler liver tissue samples isolated from
broilers were maintained for 21 days, then the examination was followed by SDS Page and Western Blott.
The results of Western Blott revealed that STAT 5b of 90 kDa had amino acid composition of
datnilvspvylypdip or aspartate, alanine, threonine, asparagine, isoleosine, leucine, valine, serine, proline,
valine, tyrosine, leucine, tyrosine, proline, aspartate, isoleucine, proline.
1 INTRODUCTION
Growth hormone has an important role in regulating
body growth and metabolism. GH metabolic effects
occur when GH receptors associate with and activate
tyrosine kinases. The bonding of GH to its receptor
can activate Janus Kinase 2 (JAK 2) and further
phosphorylate tyrosine in the JAK-2 GH-receptor
complex. This tyrosine then forms the bonding site
for a number of signalling proteins, such as signal
transducers and activators of transcription (STAT) to
induce the growth effect. STAT proteins that play a
role in providing healing signals are STAT 1, STAT
3, STAT 5a and STAT B. STAT proteins play a
significant role in regulating metabolic and growth
effects.
Increased growth in animal husbandry has great
implications and appeal to the world of poultry.
However, signalling of STAT protein and its
expression patterns in broilers during growth period
have not been identified. So, the identification of the
molecular weight and the composition of amino
acids protein signalling STAT in broilers during the
growing period due to increased GH can be used to
make synthetic STAT protein to spur the growth of
the broilers.
Until recently, it has only been known that the
weight of STAT1 protein molecules in broilers was
91 kD, while the weight of STAT 3 protein was 83
kD. Therefore, further research is needed to
determine the molecular weight of STAT 5b
proteins, as well as the amino acid composition of
STAT 5b proteins to be used as the basis for making
synthetic STAT proteins to stimulate broiler growth.
2 MATERIALS AND METHODS
The chickens were placed in a battery cage with a
capacity of one chicken per cage receiving feed
twice a day at 06.00 pm and 18.00 pm in an amount
of 10% less than standard. At the age of 21 days, the
chickens were cut to be sampled for their hepatic
tissue for the following tests: (1) Isolation of
signalling proteins STAT 5a and STAT 5b from
broiler liver tissue, (2) Analysis of STAT 5a and
STAT 5b protein signalling from broiler liver tissue
by using Dot Blot method and then continued with
SDS-PAGE (sodium dudecyl sulphate
polyacrylamide gel electrophoreses), (3)
Identification of molecular weight of signalling
protein STAT 5a and STAT 5b using Western Blot
technique by means of electrophoresis elucidated
protein from polyacrylamide gel, and (4)
Identification of amino acids by MALDI-TOF
method.
Ma’ruf, A., Damayanti, R. and Hidajati, N.
Signal Tranducers and Activators Transcription (STAT) 5b Protein as A Candidate of Growth Promoter in Broiler Chicken.
DOI: 10.5220/0007546205250527
In Proceedings of the 2nd International Conference Postgraduate School (ICPS 2018), pages 525-527
ISBN: 978-989-758-348-3
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
525
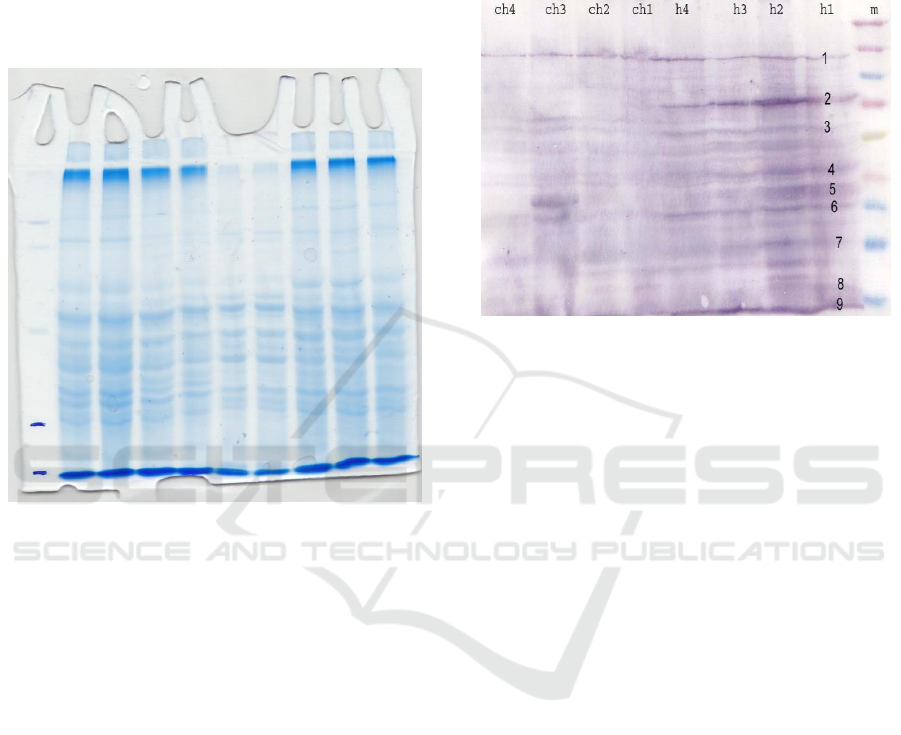
3 RESULT AND DISCUSION
3.1 SDS Page for STAT protein of the
broilers’ liver
The result of SDS-PAGE of STAT protein of broiler
chicken liver tissue showed the presence of STAT
protein as in Figure 1.
Figure 1. SDS-PAGE of STAT protein from broilers’
The results of SDS-PAGE on broilers’ liver
tissue revealed the presence of STAT protein. The
SDS-PAGE results showed that there were several
bands visible. The markers 260 with 140 kDa, 140
with 100 kDa, and 100 kDa marker with 70 kDa
each portrayed one protein band. The protein band
formed between 100 kDa and 70 kDa markers was
suspected of STAT protein. The protein bands
formed in the liver were very clear, indicating that
the liver tissue illustrationed strongest antibody
antigen reaction.
Results of SDS-PAGE on the liver tissue
proteins showed the presence of protein bands
between 100 kDa and 70 kDa markers. These were
proteins with molecular weights of 90 kDa and 91
kDa. However, there has not been able to determine
whether these results were STAT 5a and STAT 5b
proteins or not because several other protein bands
were also formed between these markers. To prove
that the formation of protein band with molecular
weight of 91 kDa and 90 kDa was indeed STAT 5a
and STAT 5b, it was necessary to make further test
using Western blot test.
3.2 Western Blot for STAT 5b protein
The result of Western blot for STAT protein on
hepatic tissue showed the presence of STAT 5b
protein with a molecular weight of 90 kDa, as in
Figure 3.
Figure 2. Western Blot for STAT 5b protein from the
broilers’
The result of calculating the weight of the STAT
5b protein molecule revealed that its weight was 90
kDa. The formation of protein bands between 100
kDa and 70 kDa markers after calculation apparently
had molecular weight of 90 kDa. This suggested that
the SDS-PAGE protein tested with Western blot was
a STAT 5b protein from growing-phase broiler with
a molecular weight of 90 kDa. The formation of a
protein band with definite 90 kDa molecular weight
was due to a bonding between STAT 5b protein
resulting from SDS- PAGE and anti-phospho-STAT
5b(Ser731).
STAT 5b with molecular weight of 90 kDa in
hepatic tissue showed that in growing phase broilers
STAT 5a and STAT 5b proteins had almost the same
molecular weight. It is expected that by identifying
the weight of the protein molecules STAT 1, STAT
3, STAT 5a and STAT 5b in broilers, researcher will
be able to determine clearly the mechanism of
growth hormone in regulating growth and
metabolism.
3.3 Amino acid of the STAT 5b proteins
The results of the MALDI-TOP examination
revealed that the STAT 5b of 90 kDa had amino acid
composition of datnilvspvylypdip or aspartate,
alanine, threonine, asparagine, isoleosine, leucine,
valine, serine, proline, valine, tyrosine, leucine,
tyrosine, proline, aspartate, isoleucine, and proline.
ICPS 2018 - 2nd International Conference Postgraduate School
526

Growth hormone plays a role in regulating body
growth and composition (Foster, 1998). Growth
hormone has a significant biologic effect that is
influenced by insulin-like growth factor I (IGF-I) in
improving skeletal muscle growth (Younken, 2000).
Provision of in vivo growth factor in broilers led to
an increase in growth rate and muscle mass by 15%
and required 6.5% less feed than normal. This
increase in growth has great implications and appeal
to the world of poultry. However, the expression
pattern of growth factor gene during growth mass to
date has not been known clearly (Killefer, 2000).
STAT protein plays an important role in the
regulation of gene transcription by GH and other
cytokines that activate Janus Kinase (JAK). STAT
proteins originally identified in the signalling
interferon pathway (IFN) (Darnell et al., 1994) are
cytoplasmic factors that contain the SH-2 domain. In
the frequent tyrosyl-phosphorylation through–the-
JAK-kinase-initiated cocktail, the cytoplasmic
STAT protein forms a complex with another STAT
protein through the phosphorylated tyrosine
interaction of the SH-2 domain, trans-locates to the
nucleus, binds to DNA and then activates
transcription of the target gene (Ihle, 1996).
Growth hormone is known to activate STATs 1,
3, 5a and 5b. Tyrosyl phosphorylation of GH-
dependent STATs 1, 3, 5a and 5b are found in 3T3-
F442A fibroblasts, in the liver of mice with
hypophysectomy, in liver cell cultures and in various
over-expression systems. Tyrosyl phosphorylation of
STATs 5a and 5b are also found in human IM-9
cells and hepatic muscle as well as skeletal muscle
of normal mice (Smit et al., 1999)
.STAT1, also called P91, is identified as a
member of the factor 3 gene complex that is
stimulated by IFN (FU, 1992). GH signalling
analysis of JAK2 deficiency cells and mutated cells
in expressing GH receptors showed that activation of
GH-dependent STATs 1, 3, 5, and 5b requires
activation of JAK2 (Smit et al., 1997). This is
consistent with the finding that JAKs activation is
required for STAT activation (Muller et al., 1993).
JAK1 or JAK2 actively overexpressed in COS cells
will stimulate the binding of STAT1 to DNA
(Silvennoinen, 1993).
An indirect study has shown that GH stimulates
the phosphorylation of STATs 1, 3 and 5 in serine or
threonine in the liver. This phosphorylation will
increase DNA binding of STAT1, and STAT3 and
substantially alter DNA binding of STAT5 (Ram
et.al., 1996). STAT 1, 3, and 5a contain conserved
consensus sequences for phosphorylation of MAP
kinases and preliminary studies show that MAP
kinase is responsible for serum phosphorylation of
STAT1, STAT3 and STAT 5a. While STAT 5b does
not contain conserved consensus sequence,
phosphorylation is performed by other kinases other
than MAP kinase. Proteins STAT 1, 3, 5a and 5b
also contain protein kinase C and casein kinase for
phosphorylation process. This suggests that double
signalling pathways may converge on STAT
proteins for transcriptional activation by GH.
4 CONCLUSIONS
The weight of the STAT 5b of 90 kDa had amino
acid composition of datnilvspvylypdip or aspartate,
alanine, threonine, asparagine, isoleosine, leucine,
valine, serine, proline, valine, tyrosine, leucine,
tyrosine, proline, aspartate, isoleucine, and proline.
Identified amino acid sequence can be used as a
basis for making STAT synthetic proteins which are
expected to be used to extend the action or effects of
growth hormone. Increased effects of growth
hormone will spur livestock growth.
REFERENCES
Darnell J, Lodish H, Baltimora D., 1990. Molecular cel
biology. 2
nd
Edition, New York: Scientific America
Books, p 715
Foster DN, Froudman JA, Harmon SA, Foster LK., 1998.
Baculovirus-mediated expression of chicken GH.
Tektran p 14
Ihle JN., 1996. Cell 84: 331-334
Kaiya H, Van Der Geyten S, Kojima M, Hosoda H,
Kitajima Y, Matsumoto M, Geeliissen S, Darras VM
and Kanagawa K. 2001. Chicken Ghrelin:
purification cDNA cloning, and biological activity.
Endocrinology 143: 3463
Killefer K, Kenny PB., 2000. Anim Vet Sci 1877.
Ram PA and Waxman DJ., 1999. J. Biol. Chem.
274:35553-35561
Ram PA, Park SH, Choi HK and Waxman DJ., 1996. J.
Biol. Chem. 271:5929-5940
Smit LS, Meyer DJ, Argetsinger LS et al., 1999
Handbooks of Physiology. Oxford University Press:
New York, pp445-480.
Younken RV, Zaou Y, Wang X et al., 2000. J Endocrinol
166:620-69
Signal Tranducers and Activators Transcription (STAT) 5b Protein as A Candidate of Growth Promoter in Broiler Chicken
527
