
Characteristics of Composite Scaffold Chitosan-Chondroitin
Sulphate / Hydroxyapatite as the Candidate of Bone Graft
Aminatun
1,2a*
; Tia Rahayu Wijayanti
1b
; Dolfi Varton
2
and Prihartini Widiyanti
1,2c
1
Department of Physics, Faculty of Science and Technology, Universitas Airlangga
Kampus C UNAIR-Jl. Mulyorejo-Surabaya-60115- Indonesia
2
Biomedical Engineering, Post Graduate School, Universitas Airlangga-
Kampus B UNAIR-Jl. Airlangga 4-6, Surabaya-60286-Indonesia
Keywords: Scaffold, Chitosan, Chondroitin Sulphate, Hydroxyapatite, Bone Graft.
Abstract: Bone defect can be addressed by using bone graft, one of them is in the form of scaffold. Tri-component
scaffold consisting of chitosan, hydroxyapatite and chondroitin sulphate is more effective for tissue
engineering since it is biocompatible, non-toxic, and biodegradable and additionally, it can be absorbed by
the body. The purpose of this study is to know the effect of adding chondroitin sulphate and to determine
the best composition of chitosan-chondroitin sulphate/hydroxyapatite as the candidate of bone graft. The
process of composite scaffold synthesis used a freeze dry method. The result of composite scaffold
chitosan-chondroitin sulphate/hydroxyapatite was obtained through characterization which covers tests on
functional group, morphology, porosity, biodegradability and cytotoxicity. The result of a functional group
test revealed that there is a stretching vibration of NH
2
on 1638.35 cm
-1
as a typical group of chitosan,
stretching vibration S=O chondroitin sulphate on 1384.41 cm
-1
, stretching vibration of P-O-C on 1089.34
cm
-1
, 1050.38 cm
-1
and 602.36 cm
-1
are the functional groups of phosphate (PO
4
3-
) hydroxyapatite. The
result of a morphology test obtained pore size range from 26-239 µm which is appropriate as the ideal
scaffold pore size. The result of a scaffold porosity test was 90.06 – 93.48% which suited the porosity of
cancellous bone. The result of a biodegradability test showed the decrease of mass on each sample which
ranged from 27.149 – 60.658% during 4 weeks. The result of a cytotoxicity test revealed that the five
samples of the composite were non-toxic toward cells. Based on these characteristics, composite scaffold
chitosan-chondroitin sulphate/hydroxyapatite has a potential to be the bone graft with the best variation on
samples with hydroxyapatite:chitosan:chondroitin sulphate composition of 50:35:15 wt%.
1 INTRODUCTION
The result of basic research by the Ministry of
Health Indonesia showed the comparison of injury
escalated in prevalence from 7.5% becoming 8.5%
from 2007 until 2013 (RISKESDAS, 2013).
Fractures happened due to traffic accidents which
reached 24 million cases per year and those caused
by osteoporosis reached 350,000 cases per year
(KEMENRISTEK, 2014). There are more than 2.2
million cases of bone-grafting in one year in all parts
of the world. In Indonesia, the growing need for
biomaterials was four times larger and the need for
bone grafts will always increase along with the
increase of bone defects caused by trauma, tumors,
congenital abnormalities, infection and so forth
(Ferdiansyah et al., 2011). Nowadays, orthopedics
mostly uses bone graft from natural bone such as
autograft, allograft dan xenograft (Darwis et al.,
2008).
Scaffold is one bone graft which is able to
provide a condition needed by cells to proliferate
and maintain each function (Humatcher and
Dietmar, 2000). Bone scaffolding is a temporal
matrix for skeletal growth and provides a specific
sphere and a construction form in regards to
developing system (Schieker et al., 2006).
Osteoblasts and chondrocyte could grow on scaffold
which will be absorbed by the body carefully and
grow as new skeletal tissue (Humatcher and
Dietmar, 2000). The ideal scaffold has three
dimensional characteristics and is porous with pore
tissue which is interconnected as a place to grow
cells and transport the flow of nutrients and
236
Aminatun, ., Wijayanti, T., Varton, D. and Widiyanti, P.
Characteristics of Composite Scaffold Chitosan-Chondroitin Sulphate / Hydroxyapatite as the Candidate of Bone Graft.
DOI: 10.5220/0007540602360243
In Proceedings of the 2nd International Conference Postgraduate School (ICPS 2018), pages 236-243
ISBN: 978-989-758-348-3
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

metabolical waste. A scaffold must be biocompatible
and bioresorbable with controllable degradation
levels and absorption levels which are suitable for
the growth of cells or tissue. Additionally, scaffold
must have mechanical characteristics which are
appropriate for tissue located in implantation areas
(Humatcher and Dietmar, 2000). Thus, the
biomaterial of bone scaffold is a potential alternative
as the improvement technique of bone defects
caused by trauma, tumor resection, and abnormal
development (Mitsak et al., 2011).
The process of making a bone graft which is
based on a composite scaffold has been conducted
by several researchers, i.e. Venkatesan et al. (2008),
by synthesizing the composite scaffold chitosan,
chondroitin sulphate, and hydroxyapatite. Chitosan
was chosen since it has some characteristics of
biocompatibility, biodegradability, and also it was
expected to be able in shaping pores and be an
appropriate media for the cells’ growth. Since the
ideal scaffold provides a suitable atmosphere for cell
proliferation, it is necessary to add materials which
could support the process of cell proliferation, such
as hydroxyapatite (HA). The biggest potential of
bone substitution indicated by HA is the ability to
build strong connection with skeletal groups, which
is osteoconductive and stable toward biological
absorption and preventing bad impacts for humans
(Orlovskii et al., 2002). The study showed that the
additional chondroitin sulphate with
collagen/hydroxyapatite caused the increase of
skeletal remodeling, new bone construction, and
osteoblast differentiation (Venkatesan et al., 2012).
Therefore, by adding chondroitin sulphate, it was
expected that the composite scaffold chitosan-
chondroitin sulphate/hydroxyapatite could be a bone
graft which could stimulate the cell growth and
accelerate the process of the skeletal remodeling
process. The research will be conducted by using
composition variations of hydroxyapatite, chitosan,
and sulphate chondroitin with comparison (A)
50%:50%:0%, (B) 50%:40%:10%, (C)
50%:35%:15%, (D) 50%:30%:20% and (E)
50%:25%:25% from the total mass. The objective of
this research is to know the effect of adding
chondroitin sulphate and determine the best
composition of composition variation of chitosan-
chondroitin sulphate/hydroxyapatite as the candidate
of bone graft.
2 RESEARCH METHOD
2.1 Materials
The materials used are commercial hydroxyapatite
produced by Tissue Bank of Dr. Soetomo Hospital
Surabaya, chitosan with 70% DA, the synthetic
result of Bogor Agricultural Institute, chondroitin
sulphate by Interlab CV, 2% acetic acid, 10% NaOH
solution, distilled water, ethanol and dehydrated
alcohol and the making of Simulation Body Fluid
(SBF) solution by using K
2
HPO
4
.3H
2
O, CaCl
2
.2
H
2
O, NaCl, NaHCO
3
, Na
2
SO
4
, KCl, HCl, MgCl
2
.6
H
2
O and (HOCH
2
)
3
CNH
2
.
2.2 The Synthesis of Scaffold
Composite of Chitosan-Chondroitin
Sulphate/ Hydroxyapatite
The solution of chitosan-chondroitin sulphate/
hydroxyapatite which has been prepared was moved
into a pot bottle. To create a scaffold, the solution
was frozen at -80
o
C temperature for 5 hours. After
that a process named freeze-drying was done to the
frozen solution for 30 hours.
After the freeze-drying process, the sample of
composite scaffold chitosan-chondroitin
sulphate/hydroxyapatite was marinated in 10%
NaOH solution for 24 hours to neutralize the acetic
acid residual present in the sample. After that, it was
cleansed by using equades until it reached the
neutral pH. Next, freeze-dying was done once again
to relieve the water wastes in the composite sample
of chitosan-chondroitin sulphate/hydroxyapatite
scaffold.
The next process is the characterization covering
functional group testing with a Fourier Transform
Infra-Red (FTIR) spectrophotometer American
Perkin Elmer Co, morphological surface testing by
using a Scanning Electron Microscope (SEM)
inspect S50, FEI Corp., porosity test,
biodegradability and cytotoxicity test by using MTT
assay.
Porosity test was done by using a fluid
displacement method. During the test, the sample of
composite scaffold which would be used was
initially weighed to find the initial weight of the
sample. After that, the sample was marinated in 98%
ethanol for 48 hours. After marinating, the scaffold
sample was re-scaled along with the ethanol to find
the weight of marinated scaffold in ethanol. The last
step was measuring the ethanol whose sample has
been taken over. The final result of porosity testing
was acquired from the initial weight of the scaffold
(w
1
), the weight of scaffold and ethanol which
are being marinated (w
2
), and the final weight of
ethanol after the scaffold was taken over (w
3
). Then,
the percentage of porosity of each composite
Characteristics of Composite Scaffold Chitosan-Chondroitin Sulphate / Hydroxyapatite as the Candidate of Bone Graft
237
scaffold sample was then calculated by using
Equation 1.
(1)
Biodegradability in-vitro test was done by
marinating the composite scaffold sample in a
Simulated Body Fluid (SBF) solution. In this test,
the composite scaffold sample was immediately
measured to see the basic weight of the scaffold.
Further, the sample of composite scaffold was
marinated in a Simulated Body Fluid (SBF) solution
for 4 weeks. The data of the biodegradability in-vitro
testing result was collected on 1
st
, 2
nd
, 3
rd
, and 4
th
week. In each data collection, the scaffold sample
was dried initially, a while later it was weighed to
reveal its final weight after being marinated. The
data acquired from the test was in the form of the
initial weight (w
0
) of each composite scaffold and
the final weight (w
1
) after marinating process. The
percentage of degradation or lost mass acquired
from the data was calculated by using Equation 2.
(2)
The cytotoxicity testing was conducted by using
MTT Assay consisting of tetrazolium salt [3-(4,5-
dimetiltiazol-2-yl)-2,5-difeniltetrazolium bromide].
The systematic principal of the MTT Assay method
is following the ability to live of the living cell based
on mothocondrial activities of cell culture. The
changes happened on tetrazolium salt [3-(4,5-
dimetiltiazol-2-yl)-2,5-difeniltetrazolium bromide]
which became formazan in an active mothocondria
as the base of the MTT Assay method. The living
cell will change MTT which was then cracked
through the reduction of reductase enzym in a chain
of mothocondrial respiratory system to formazan
which was dissolved in purple. The bigger the
absorbance, the more the living cells calculated by
using Equation 3.
(3)
where ODT = OD treated cells, ODM = OD media
control and ODC = OD cells control.
3 RESULTS AND DISCUSSION
3.1 The Analysis of Functional Group by Using Fourier Transform Infra-Red (FTIR)
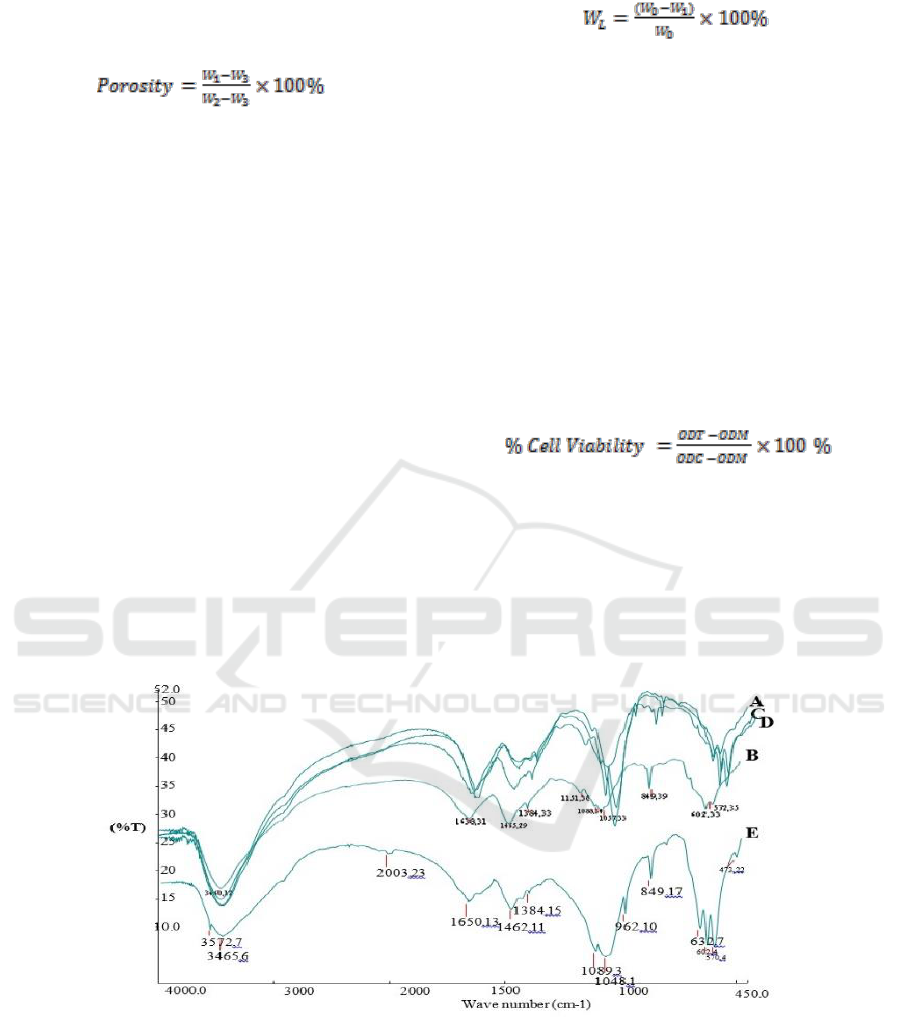
Figure 1: FTIR Spectrum of Composite Scaffold Chitosan-Chondroitin Sulphate / Hydroxyapatite.
Based on Figure 1, the result of FTIR test, it was
acquired wavenumber as the stretching vibration of
O-H, the O-H of all materials consisting of OH
functional group. The absorption area on the
wavenumber 1638.35 cm
-1
was the stretching
vibration of Amina (NH
2
) and carbonyl (C=O)
functional group. The wavenumber of 1459.39 cm
-1
is the bending vibration of C-H from CH
2
group. On
each absorption, the wavenumber 1384.41 cm
-1
is
the stretching vibration of S-O, the characteristic of
SO
3
-
functional group as the group of chondroitin
sulphate. The stretching vibration of P-O-C was
shown by the absorption area on the wavenumber
1089.34 cm
-1
, 1050.38 cm
-1
and 602.36 cm
-1
ICPS 2018 - 2nd International Conference Postgraduate School
238
included in the phosphate functional group (PO
4
3-
),
the specification of hydroxyapatite. The carbonate
functional group (CO
3
2-
) found on the absorption
band on wavenumber 849.49 cm
-1
. Besides, it was
the distinctive characteristic of the chondroitin
sulphate absorption band. The absorption band on
the waveband 571.35 cm
-1
belongs to
hydroxyapatite. The carbonate functional group
(CO
3
2-
) appearing in the result of FTIR test was
originally from the absorption of carbon dioxide
from the atmosphere.
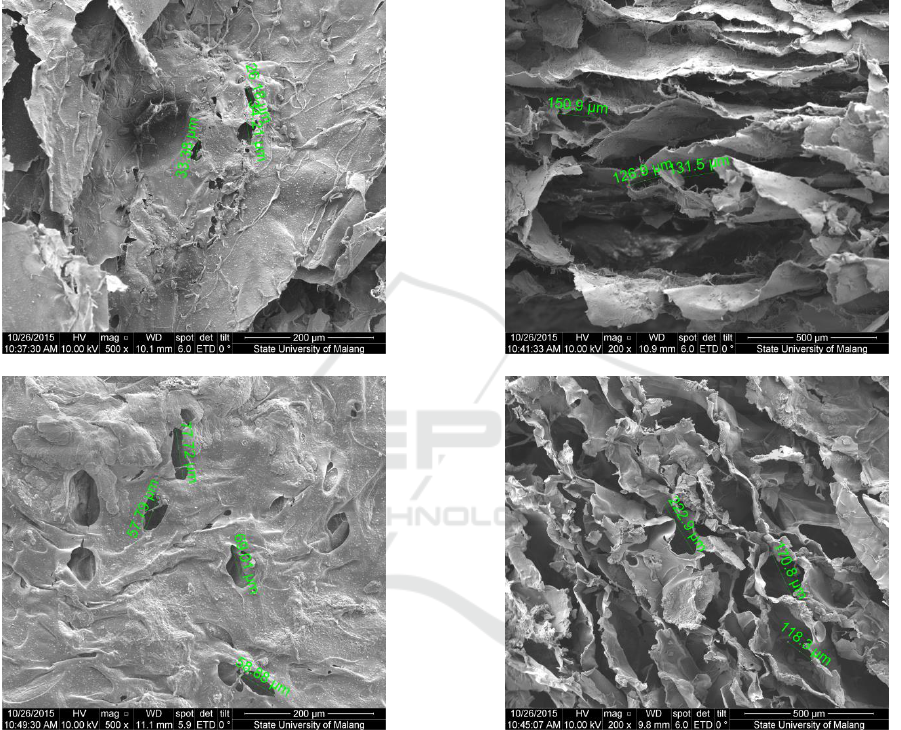
3.2 Morphology Test
a
b
c
d
Characteristics of Composite Scaffold Chitosan-Chondroitin Sulphate / Hydroxyapatite as the Candidate of Bone Graft
239
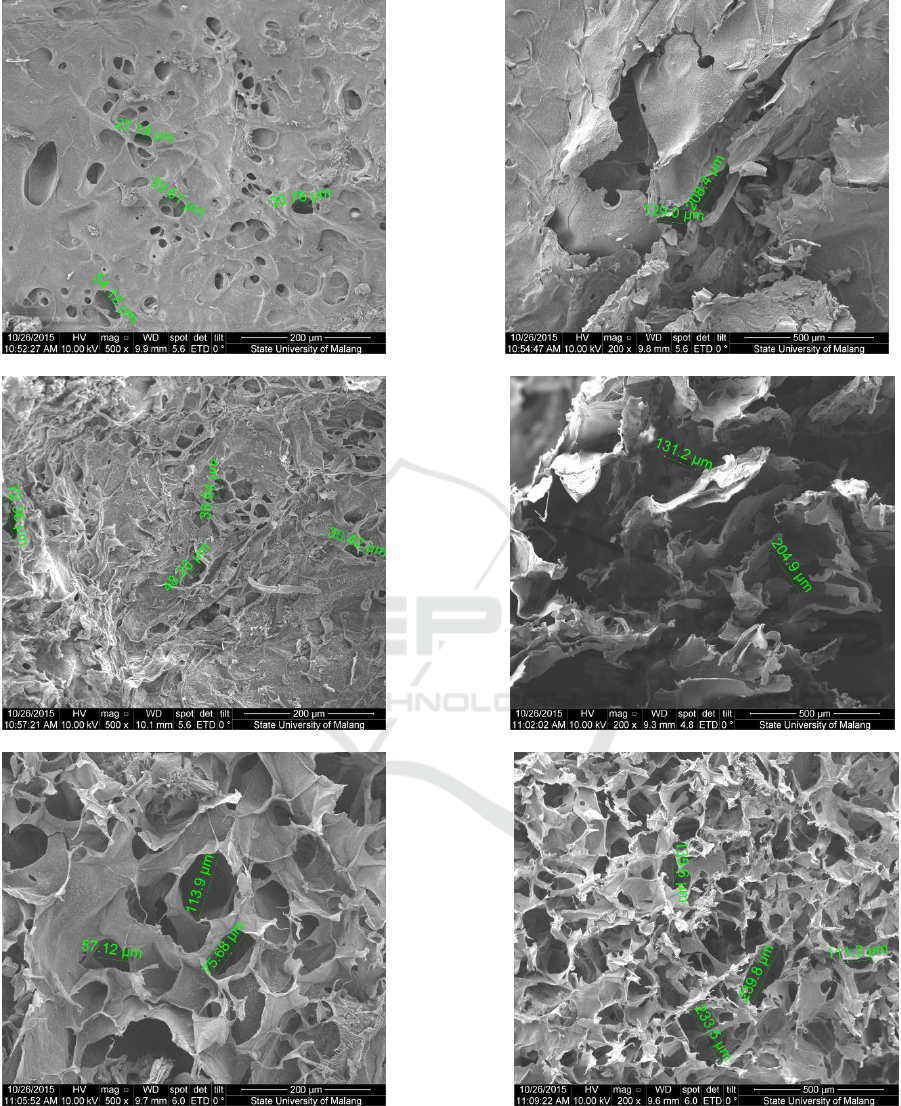
Figure 2: Results of Morphology Test of Composite Scaffold Chitosan-Chondroitin Sulphate/Hydroxyapatite with the
variation composition of (a)50:50:0 wt% transversal, (b) 50:50:0 wt% vertical, (c) 50:40:10 wt% transversal, (d) 50:40:10
wt% vertical, (e) 50:35:15 wt% transversal (f) 50:35:15 wt% vertical, (g) 50:30:20 wt% transversal (h) 50:30:20 wt%
vertical, (i) 50:25:25 wt% transversal dan (j) 50:25:25 wt% vertical
e
f
g
h
i
j
ICPS 2018 - 2nd International Conference Postgraduate School
240
The observation on morphology test was done on
the sample by seeing the transversal and vertical
longitudinal plane with 200x and 500x enlargement.
The measurement of pore on the scaffold sample
was done by using a software available in SEM. A
ruler was used to meter the diameter of pore seen
from the smallest and biggest diameter on the
picture as the result of SEM.
A very porous scaffold facilitated the seeding
and immigration of cells while the smaller pores
enable the growth of tissue. Based on data in Table
1, it was acquired the A, B, C, D, and E samples
have fulfilled the criteria of diameter ideal size of a
scaffold’s pore to help the process of
osteoconduction on range 200-350 µm. Meanwhile,
the most suitable criteria was on sample C since it
has the closest range of diameter size to the pore size
of the scaffold which could assist the growth of
fibroblast and osteoconduction.
Table 1: Pore Size of Composite Scaffold Chitosan-
Chondroitin Sulphate/Hydroxyapatite.
Samples:
Hydroxyapatite:
Chitosan:Chondroitin
Sulphate (wt%)
Pore Size (µm)
A (50:50:0)
26 - 150
B (50:40:10)
57 - 223
C (50:35:15)
27 - 208
D (50:30:20)
30 - 205
E (50:25:25)
57 - 239
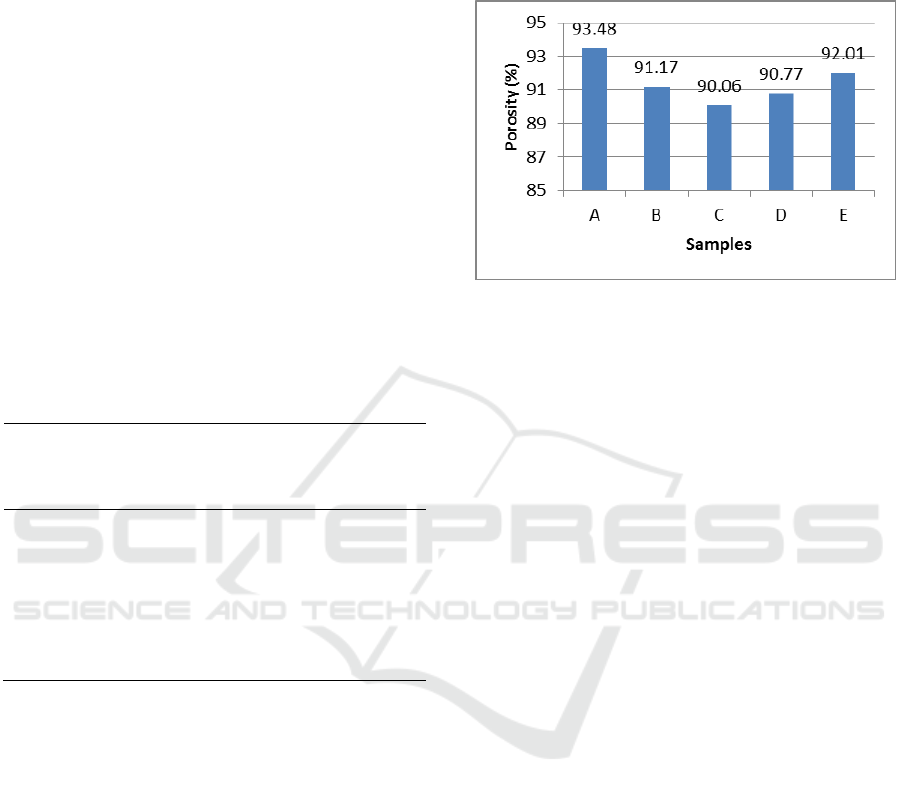
3.3 Porosity Test
The porosity of a scaffold has an essential role in
regenerating tissue. It provides the temporal
mechanical function and facilitates the migration of
cells. High porosity (90%) was chosen for various
scaffold designs since it is possible to diffuse
adequate nutrition during the tissue growing and
provide sufficient area for cells and biomaterial to
interact with each other. The biggest percentage of
porosity (Figure 3) was on sample A, the composite
scaffold of chotisan/hydroxyapatite with 50:50%
composition compared to samples B, C, D, and E by
adding variation of chondroitin sulphate composition
40%wt, 35%wt, 30%wt, and 25%wt each. The
declined percentage of porosity of sample B, C, D,
and E, if compared to sample A, happened due to the
additional chondroitin sulphate on the composite
scaffold sample causing an intermolecular knot of
hydrogen between chitosan and hydroxyapatite.
From the results of the porosity test using a fluid
displacement method, it can be concluded that
sample C was the best result with porosity
percentage of 90.06%.
Figure 3: Porosity Percentage Samples of Composite
Scaffold Chitosan-Chondroitin Sulphate/Hydroxyapatite
3.4 Biodegradability In Vitro Test
The ideal scaffold has a controllable degradation
level which suits the skeletal reparation process. The
purpose of a biodegradability in vitro test on a
composite scaffold sample is to know the level of
composite scaffold biodegradation in the
environment of body fluid. The result of a
biodegradability in vitro test using SBF fluid
increased the degradation level from the first until
the fourth week. In the fourth week, it can be seen
that composite scaffold still has not been degraded
thoroughly, so that it was not good enough for the
development of skeletal tissue. While vascular
development was in progress, the collagen matrix
was secreted by osteoid then mineralized, which
directed the formation of soft callus around the
reparation area. The callus is broken in 4-6 weeks
from the recovery process and needed adequate
protection in the form of bracing or internal fixation.
Thus, it can be concluded that after 4 weeks, the
composite scaffold chitosan - chondroitin sulphate
/hydroxyapatite could still provide space for the
growth of skeletal tissue so that it could increase in
vitro bioactivity on the scaffold, with sample C as
the best result with lost mass of 27.1485% during 4
weeks (Figure 4).
Characteristics of Composite Scaffold Chitosan-Chondroitin Sulphate / Hydroxyapatite as the Candidate of Bone Graft
241
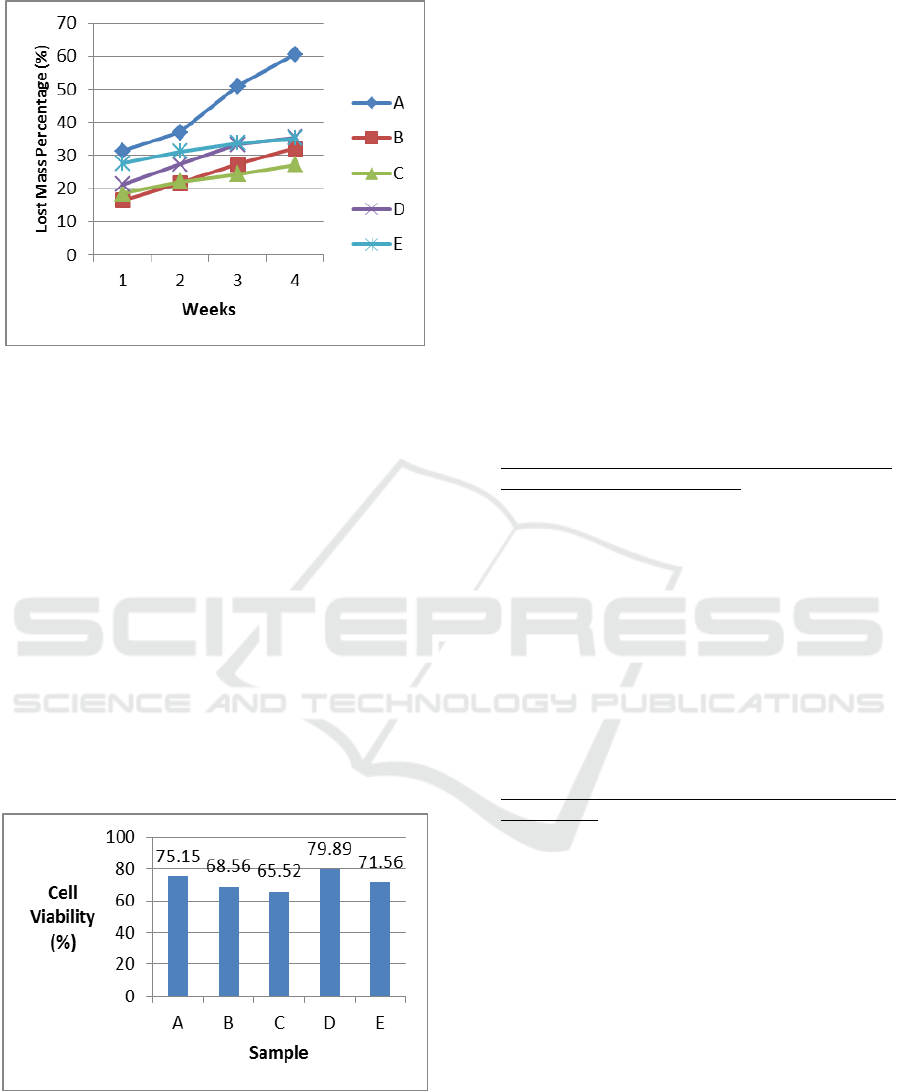
Figure 4: Graphic of Lost Mass Percentage of Samples
Composite Scaffold Chitosan-Chondroitin
Sulphate/Hydroxyapatite
3.5 Cytotoxicity Test
Cytotoxicity test aims at knowing the nature of
cytotoxicity in a sample of composite scaffold
chitosan-chondroitin sulphate/hydroxyapatite toward
the living cell perceived from the viability cell or
living cell percentage. A material is called non-toxic
if the percentage of cell viability is more than 50%
(Spielmann et al., 2007). On the cytotoxicity test
using MTT Assay on the composite scaffold sample
A, B, C, D, and E, it obtained the percentage of the
living cell as high as 75.15%, 68.56%, 65.52%,
79.89 and 71.56% (Figure 5). This shows that by
adding chondroitin sulphate on the composite
scaffold sample, the sample became intoxicating for
the living cell.
Figure 5: Cell Viability of Composite Scaffold Chitosan-
Chondroitin Sulphate /Hydroxyapatite
4 CONCLUSIONS
Composition variation of chondroitin sulphate
affects the pore size, porosity, and the percentage
of lost mass on a composite scaffold. Composite
scaffold chitosan - chondroitin
sulphate/hydroxyapatite can be used as the
candidate of bone graft. The best result was
acquired from sample C (35:15:50 wt%) with pore
size range 27-208µm, porosity 90.06%, lost mass
percentage was 27.187% for 4 weeks, and cell
viability was 65.52%.
REFERENCES
Badan Penelitian dan Pengembangan Kesehatan
Kementrian Kesehatan RI. 2013. Riset Kesehatan
Dasar (RISKESDAS 2013). Downloaded from:
http://www.litbang.depkes.go.id/sites/download/rkd20
13/Laporan_Riskesdas2013.PDF on 10 December
2014 at 06.09 WIB.
Darwis, Darmawan, dan Yessy Warastuti. 2008. Sintesis
dan Karakterisasi Komposit Hidroksiapatit (HA)
Sebagai Graft Tulang Sintetik. Jurnal Ilmiah Aplikasi
dan Radiasi Vol 4 No.2 Desember 2008.
Ferdiansyah, Djoko Rushadi, Fedik Abdul Rantam,
Aulani’am. 2011. Regenerasi pada Massive Bone
Defect dengan Bovine Hydroxyapatite sebagai Scaffold
Mesenchymal Stem Cell. JBP Vol 13, No.3.
Hutmacher, Dietmar W. 2000. Scaffolds in tissue
engineering bone and cartilage. Elsevier B.V:
Biomaterials 21 (2000) pp. 2529-2543.
Kementrian Riset dan Teknologi Republik Indonesia.
2014. Accessed on November 2014 at 17.58 WIB in:
http://www.ristek.go.id/index.php/module/News+News
/id/12533/pdf
Mitsak, Anna G, Jesscia M. Kemppainen, Matthew T,
Harris, Scott J Hollister. 2011. Effect of
Polycaprolactone Scaffold Permeability on Bone
Regeneration in Vivo. Michingan: Tissue Engineering:
Part A, Vol 17, Numbers 13 and 14.
Orlovskii, V.P., V. S. Komlev, dan S. M. Barinov. 2002.
Hydroxyapatite and Hydroxyapatite-Based Ceramics.
Russia: Inorganic Materials, Vol. 38, No. 10, 2002,
pp.973-984.
Schieker, Matthias, Herman Seitz, Inga Drosse, Sebastian
Seitz, Wolf Mutschier. 2006. Biomaterials as Scaffold
dor Bone Tissue Engineering. German: European
Journal of Trauma 2006 No.2.
Spielmann, H. Hoffman, S. Botham, P. Roguet, R. Jones,
P. 2007. The ECVA International Validation Study on
In Vitro Test for Acute Skin Irritation Report on the
Validity of the EPISKIN and EpiDerm Assays on the
Skin Integrity Function Test. Germany: ATLA.
Venkatesan, Jayachandran, Ramjee Pallela 2012.
Chitosan-amylopectin/hydroxyapatite and chitosan-
chondroitin sulphate/hydroxyapatite composite
ICPS 2018 - 2nd International Conference Postgraduate School
242

scaffolds for bone tissue engineering. International
Journal of Biological Macromolecules 51 (2012)
halaman 1033-1042.
Characteristics of Composite Scaffold Chitosan-Chondroitin Sulphate / Hydroxyapatite as the Candidate of Bone Graft
243
