
Preparation of TiO2/RGO Composite and Application in
Photocatalytic Degradation of Unsymmetrical Dimethylhydrazine
Wastewater
Xiaomeng Lv
1
, Xuanjun Wang
1
, Ying Jia
1
, Zhiyong Huang
1
and Qilong Han
1
1
Rocket Force University Of Engineering
,
Xi
’
an
,
P.R. China
Keywords: TiO
2
/RGO, Preparation, UDMH wastewater, Photocatalytic Degradation.
Abstract: The TiO
2
/reduced graphene oxide composite was prepared with graphene oxide and titanium dioxide by
hydrothermal reduction method. Surface morphologies were collected by scanning electron microscopy and
ultraviolet-visible absorption performance was studied by ultraviolet-visible spectrophotometer. The
TiO
2
/RGO composites were used as catalysts for photocatalytic degradation of unsymmetrical
dimethylhydrazine wastewater with a concentration of 120 mgꞏl
-1
. The optimum condition of catalyst dosage,
pH value and reaction time were 1gꞏl
-1
, 7 and 120 min respectively and the optimum photocatalytic
degradation rate of UDMH wastewater was 72.1%.
1 INTRODUCTION
Unsymmetrical dimethylhydrazine (UDMH) is an
important liquid propellant used in aerospace and
military field. It is difficult to achieve completely
photocatalytic degradation of UDMH in wastewater
disposal with single catalyst owing to its
complicated molecular structure. Thus, searching for
composite catalytic materials for the photocatalytic
degradation of UDMH wastewater is important.
New carbon materials such as graphene and reduced
graphene oxide (RGO) et al. have a promising
application prospects in flexible graphite, thermal
energy storage materials, sorbents, conductive resin
composites and catalyst[1-2]. As a wide-band gap
semiconductor material, TiO2 particle has good
chemical stability, thermal conductivity, and
ultraviolet absorption that be applied in
photoelectron apparatus and photocatalyst[3-4].
Hybridization reaction of TiO2 particle and RGO
was conducted easily owing to a few oxygen-
containing function groups on the surface of RGO.
In the present study, TiO2/RGO composites were
prepared with graphene oxide and titanium dioxide
as raw materials by hydrothermal reduction method.
Then, TiO2/RGO applied in the photocatalytic
degradation experiment on UDMH wastewater was
carried out and the influence of reaction condition
on the optimum photocatalytic degradation rate was
also discussed.
2 EXPERIMENTAL
2.1 Preparation of TiO
2
/RGO
An appropriate amount of 50 mg (to the accuracy of
0.1 mg) graphene oxide (GO) sample was placed
into a with 50 ml NaOH solution of 10 mol•l-1.
After 2 hours dispersion treatment of the beaker in
ultrasonic processor, a certain amount of TiO2
particle was added and then another 2 hours
dispersion treatment was conducted. Dispersed
solution was placed into a high pressure resistant
reaction container, magnetic stirred in room
temperature for 4 hours, heated in 150 °C for 24
hours, removed and washed with dilute hydrochloric
acid and ultrapure water successively, and then
placed into a vacuum drier of 60 °C for 12 hours and
a vacuum tube furnace of 600 °C (pure nitrogen
environment) for 1 hour. Then, TiO2/RGO
composites were obtained.
2.2 Characterization
Surface morphologies of TiO2/RGO composites
were collected by scanning electron microscopy
(Czech, TESCAN, VEGA ІІ XMUINCN).
Ultraviolet-visible (UV-vis) absorption spectrum
was collected by UV-vis spectrophotometer (Japan,
SHIMADZU, UV-2700, 200 nm ~ 900 nm).

2.3 Photocatalytic Degradation of
UDMH Wastewater
TiO
2
particles and TiO
2
/RGO composites were used
as catalysts for photocatalytic degradation
experiment of 120 mg•l
-1
UDMH wastewater. A
self-made ultraviolet light photocatalytic device as
shown in Figure 1 was constituted of ultraviolet light
lamp, sealed wooded cases and strong magnetic
stirrer. The beaker containing UDMH wastewater
was placed under the UV lamp and the liquid level
of UDMH wastewater was 10 cm far away from the
UV lamp. The experiment of influence of catalyst
dosage, pH and reaction time on the photocatalytic
degradation rate was carried out.
Figure 1: A self-made UV light photocatalytic device.
3 RESULTS AND DISCUSSION
3.1 SEM Analysis
Figure 2: SEM images of TiO
2
/RGO.
Figure 2 shows SEM images of TiO
2
/RGO
composites prepared that with high purity have well-
proportioned size in the range from 5 μm to 30 μm.
Surface of TiO
2
/RGO composites appearing
spherical particles was covered with fold thin RGO
film. As shown in Figure 2, TiO
2
/RGO composites
with small agglomeration were clearly observed.
This result indicates that the oxygen-containing
function groups on the surface RGO attained
become fewer after reduction and the hydrophilicity
of RGO become lower. After Hybridization reaction
of TiO
2
particle and RGO, agglomeration of
TiO
2
/RGO could be restrained effectively owing to
its own nanostructure.
3.2 UV-vis Analysis
A comparative study of UV-vis absorption spectrum
analysis between TiO
2
particles and TiO
2
/RGO
composites were studied by UV-2700 ultraviolet-
visible spectrophotometer as shown in Figure 3. The
result shows a marked growing of UV-vis absorption
intensity of TiO
2
/RGO in the range from 200 nm to
400 nm. The UV-vis absorption intensity of
TiO
2
/RGO-1 was 80% stronger than that of TiO
2
.
Owning to sufficient electron transfer from valence
band to conduction band, most transfer electrons
were accepted by RGO and few electrons back was
recombined with hole. The more the electron holes
mount exist on the surface of TiO
2
/RGO, the
stronger the catalytic oxidation will be.
Figure 3: UV-vis absorption spectrum of TiO
2
/RGO.
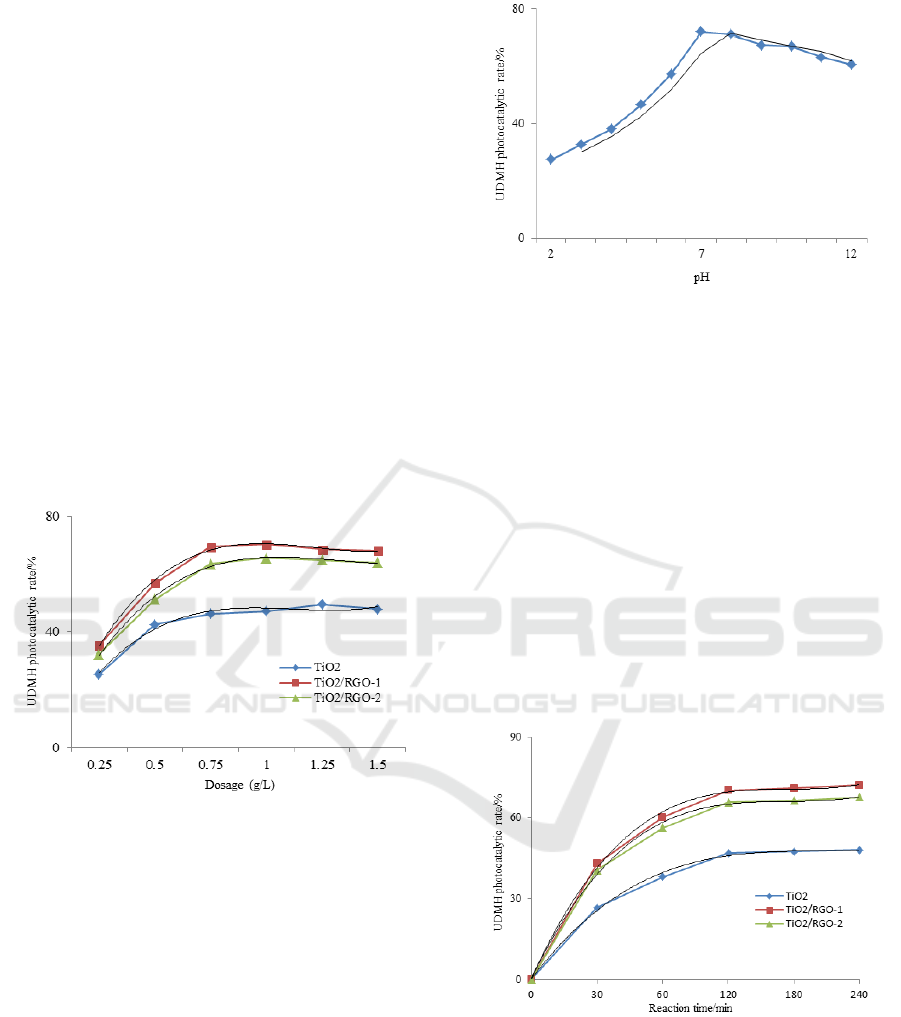
3.3 Photocatalytic Property Analysis
Figure 4 shows the result of influence of catalyst
dosage on photocatalytic degradation rate of
120mg•l
-1
UDMH wastewater with TiO
2
and
TiO
2
/RGO samples. The increase in catalyst dosage
from 0.25 g•l
-1
to 0.75 g•l
-1
resulted in an accelerated
photocatalytic degradation rate of UDMH
wastewater. The photocatalytic degradation rate
increased to its maximum at about catalyst dosage of
1 g•l
-1
, and then decreased slowly because of more
catalyst dosage, possibly resulting in reducing UV-
vis absorption of TiO
2
and TiO
2
/RGO.
The degradation rate of UDMH wastewater with
TiO2/RGO catalyst can achieve 70.2% that was
approximately 50% higher than that of TiO2 particle.
This result indicates that Hybridization reaction is
advantageous to increase of the specific area and
absorption of TiO2/RGO. This condition could
result in improving surface chemical mobility and
broadening energy band gap of TiO2/RGO, and in
favor of the increase of degradation rate.
Figure 4: Influence of catalyst dosage on photocatalytic
degradation rates of UDMH wastewater.
The results of influence of pH value on
photocatalytic degradation rate of 120 mg•l
-1
UDMH
wastewater with TiO
2
and TiO
2
/RGO samples were
shown in Figure 5. The increase in pH value from 2
to 8 resulted in a gradual growing photocatalytic
degradation rate of UDMH wastewater from 27.5%
to 72.1%. The photocatalytic degradation rate
increased to its maximum at about pH value of 7,
and then decreased gradually.
Figure 5: Influence of pH value on photocatalytic
degradation rates of UDMH wastewater.
Because of physicochemical property of UDMH,
the solution pH value is a very important factor for
photocatalytic degradation experiment. A
neutralization reaction with unsymmetrical
dimethylhydrazine and hydrogen ion occurred and
UDMH-salt compounds were generated in acid
condition. TiO
2
/RGO was photocatalytic-insensitive
to UDMH-salt compounds, which resulting in a
lower photocatalytic degradation rate. In neutral and
alkalescent condition, UDMH existed in molecular
form that could be benefit to photocatalytic
degradation. The pH value above 9 is advantageous
for electron transfer due to high concentration of
hydroxide and the photocatalytic reaction with
TiO
2
/RGO and UMMH could be restrained partly
due to decrease of electron-hole pair amount.
Figure 6: Influence of reaction time on photocatalytic
degradation rates of UDMH wastewater.
Figure 6 shows the result of influence of reaction
time on photocatalytic degradation rate of 120 mg•l
-1
UDMH wastewater with TiO
2
and TiO
2
/RGO
samples. As the reaction time increased, the
photocatalytic degradation rate increased at the
initial reaction and became closer to its maximum
72.1% at 120 min. This result indicates that

photocatalytic reaction was completed after 120 min.
In the initial reaction time from 0 min to 60 min, a
significant increase of photocatalytic degradation
rate occurred owing to large specific area of
TiO
2
/RGO and rapidly absorption to UDMH
molecular that was benefit to photocatalytic reaction.
The reaction time longer than 120 min is
disadvantageous for the increase in photocatalytic
degradation rate because of absorption-degradation
saturation balance between TiO
2
/RGO and UDMH
molecular.
4 CONCLUSIONS
The TiO
2
/RGO composite was prepared with
graphene oxide and titanium dioxide as raw
materials by hydrothermal reduction method. The
TiO
2
/RGO composites were used as catalysts for
photocatalytic degradation of unsymmetrical
dimethylhydrazine wastewater with a concentration
of 120 mg•l
-1
. The optimum condition of catalyst
dosage, pH value and reaction time were 1g•l
-1
, 7
and 120 min respectively and the optimum
photocatalytic degradation rate of UDMH
wastewater was 72.1%.
REFERENCES
1. XIAO X Y, LI Y C, LIU Z P. Graphene
commercialization [J]. Nat Mater, 2016, 15: 697-698.
2. Li J H5 Li J Y, Li L F, et al. Flexible graphene fibers
prepared by chemical reduction induced self-assembly
[J]. Journal of Materials Chemistry A, 2014, 2(18):
6359-6362.
3. J.S. Lee, K.H. You, C.B. Park, Highly Photoactive,
Low Band gap TiO2 Nanoparticles Wrapped by
Graphene [J]. Advanced Materials 24 (2012): 1084-
1088.
4. Y.Y. Gao, X.P. Pu, D.F. Zhang, G.Q. Ding, X. Shao, J.
Ma, Combustion synthesis of grapheneoxide-TiO2
hybrid materials for photodegradation of methyl
orange, Carbon 50 (2012):4093-4101.
