
Effects of Minor Zirconium on Microstructure and Mechanical
Properties of Al-Mg-Mn-Sc Alloys
Xianming Chen
1
, Yingying Fan
1
and Qinglin Pan
2
1
School of Electronic and Electrical Engineering, Zhaoqing University, Zhaoqing, 526061, China
;
2
School of Materials Science and Engineering, Central South University, Changsha, 410083, China
Keywords: Al-Mg-Mn-Sc alloys; Al3Sc;Al3(Sc,Zr);microstructure and mechanical properties; Al-Mg-Mn-Sc-Zr
alloys
Abstract: Al-5.8Mg-0.4Mn-0.25Sc and Al−5.8Mg−0.4Mn−0.25Sc−0.1Zr (mass fraction, %) alloys were prepared by
water chilling copper mould ingot metallurgy processing which was protected by active flux. The effects of
Zr on microstructure and mechanical properties were studied by means of observations of optical
microscopy and transmission electron microscopy. The results show that after adding Zr into Al-5.8Mg-
0.4Mn-0.25Sc alloy the grains are obviously refined. Tensile properties increase significantly; under the
condition that ductility(δ) is similar, tensile strength(σb) increases by nearly 20%, and the yield
strength(σ0.2) increases by more than 30%. Zirconium in the alloy leads to the formation of more
heterogeneous nucleation core during casting process, and more primary Al3(Sc,Zr) particles precipitate,
thus refining the grain. In the same time the supersaturated solid solubility in the alloy can be improved.
After stabilizing annealing, there is a higher degree of dispersion, and smaller and more secondary
Al3(Sc,Zr) particles come to distribute in the matrix, improving the comprehensive mechanical properties of
alloy with functions of strong pinning dislocations and sub-grain boundaries, and stabilizing sub-structures.
1 INTRODUCTION
Al-Mg-Mn belongs to the moderately strong,
corrosion-resistant, weldable aluminum alloy.
Because of their excellent comprehensive
performance, they have been widely used in
aerospace, transportation, electronic appliances,
instruments and meters, armed ships, etc[1]. With
the development of technology and increasingly
diverse application requirements, higher
requirements on the performance of these alloys
have put forward. This kind of alloy cannot be
strengthened by heat treatment. One way to increase
the properties is by improving the deformation
process; the other way is through micro alloying.
The studies show that in improving Al-Mg-Mn alloy
performance effect is prominent[2-4]. In all of the
adding elements, the most significant element is Sc.
Therefore in Russia a series of scandium aluminum
alloy have been researched and developed depend on
Sc, such as 01570 and 01571 alloys. Adding Sc into
Al-Mg-Mn alloy, the primary Al
3
Sc phase will
precipitate during casting process, dendritic structure
will be removed; and the grain size will be refined.
And in the process of cold and hot deformation and
stabilizing treatment, secondary Al
3
Sc particles will
precipitate, improving the performance of the alloy
greatly with pinning dislocation, sub-grain boundary,
and stable sub-structure.
In order to excavate the potential of Sc as much
as possible, and to improve the performance of alloy
more significantly, the research is focused on the
composite micro alloying in recent, such as Sc and
Zr[5-6], Sc and Ti
[
7-8], Sc and Er[9-10], Sc, Zr and
Ti
[11]
. The study finds out that the effect of adding
Sc and Zr together into Al-Mg-Mn alloy is better
than that of adding single Sc. meanwhile, the
strength properties and the recrystallization
temperature are higher[1]. But the research of
composite micro alloying about Sc and Zr is more
focused on the role of the Sc, and the work about Zr
may not be enough. In this work, both Al-5.8Mg-
0.4Mn-0.25Sc and Al-5.8Mg-0.4Mn-0.25Sc-0.1Zr
alloys are used as the research objects. The aim of
this work is to investigate the role and the effecting
rule of Zr to Al-Mg-Mn-Sc alloy.

2 EXPERIMENTAL
Two alloys for the study (marked A, B) are prepared
by ingot metallurgy, using as the starting materials
pure Al, pure Mg and Al-2.23%Sc, Al-4.48%Zr, Al-
8.5%Mn master alloys. Their nominal compositions
are listed in Table 1. After homogenization at 460°C
for 24h, the ingots are cut head and milled surface to
25mm thickness. Then a hot-rolling process is
applied to 6mm after heat preservation for 3h at
470 °C, which is followed by intermediate annealing
at 400 °C for 2h. Subsequently, the hot-rolled sheets
are cold-rolled to a thickness of 2.0 mm. The total
deformation rate is up to 92%. The cold-rolled
sheets are annealed at 340 °C or 150°C for 1h.
Homogenization; intermediate annealing and
stabilizing annealing processing are made in SPC
box-type resistance furnace, and the error is ±2 ℃.
The microstructures of the alloys are examined
using a POLYVER-Met optical microscope, with the
specimens first mechanical polished, then electro-
polished, followed by anodizing in a water solution
of HF and H
3
BO
3
(30mlHF+11gH
3
BO
3
+970mlH
2
O).
Electrolytic polishing voltage is 20~28V, about 1~3
min, and anodizing voltage is 15~25, about 1~2min.
TEM thin foils are prepared by twin-jet polishing
with an electrolyte solution composed of 30%HNO
3
and 70%CH
3
OH(volume fraction) at the temperature
below -25°C. The foils are examined using
HITACHI-800 and TECNAI G220 transmission
electron microscope at an accelerating voltage of
200kV. Tensile specimens are cut along the rolling
direction of the plates and tested on a MTS-858
tensile testing machine according to GB/T 228-2002
standard.
Table 1: Chemical composition of the studied alloys.
Specimen
No.
Chemical composition
(mass fraction, %)
Al Mg Mn Sc Zr
A Bal. 5.8 0.4 0.25
B Bal. 5.8 0.4 0.25 0.1
3 RESULTS
3.1 Effect of Minor Zr on the Tensile
Properties of Al-Mg-Mn-Sc Alloys
Table 2: Tensile properties of two studied alloys
Heat treatments
σb , MPa
A B
130℃/1h 396 472
340℃/1h 336 395
Heat treatments
σ0.2 , MPa
A B
130℃/1h 304 406
340℃/1h 191 268
Heat treatments
δ,%
A B
130℃/1h 11.4 9.7
340℃/1h 15.5 16.5
Table2 lists tensile properties of the 2 alloys
annealed at 130 °C or 340°C for 1h. It is clear that
co-addition of small amounts of Sc and Zr can have
higher tensile strength(σ
b
) and higher yield
strength(σ
0.2
) than that of adding single Sc. Under
the condition that ductility(δ) is similar, tensile
strength increases by nearly 20%, and the yield
strength increases by more than 30%. It can also be
seen in the table 2 that two kinds of alloy
mechanical properties and annealing system have
close relations. In order to maintain higher ductility,
340℃/1h annealing system is a better choice. The
experimental results show that Zr plays a great role
in improving the mechanical properties of Al-Mg-
Mn-Sc alloy.
3.2 Effects of Trace Zr on the Optical
Microstructure
Figure1 illustrates the optical microstructures of the
2 alloys in different states. It is shown that in 2
alloys dendritic structures haven’t been
found(Figure.1a, b). These observations indicate that
addition of 0.2%Sc alone, or co-addition of 0.25%Sc
and 0.1%Zr into the Al-Mg-Mn alloys could bring
about an inoculation effect of the cast
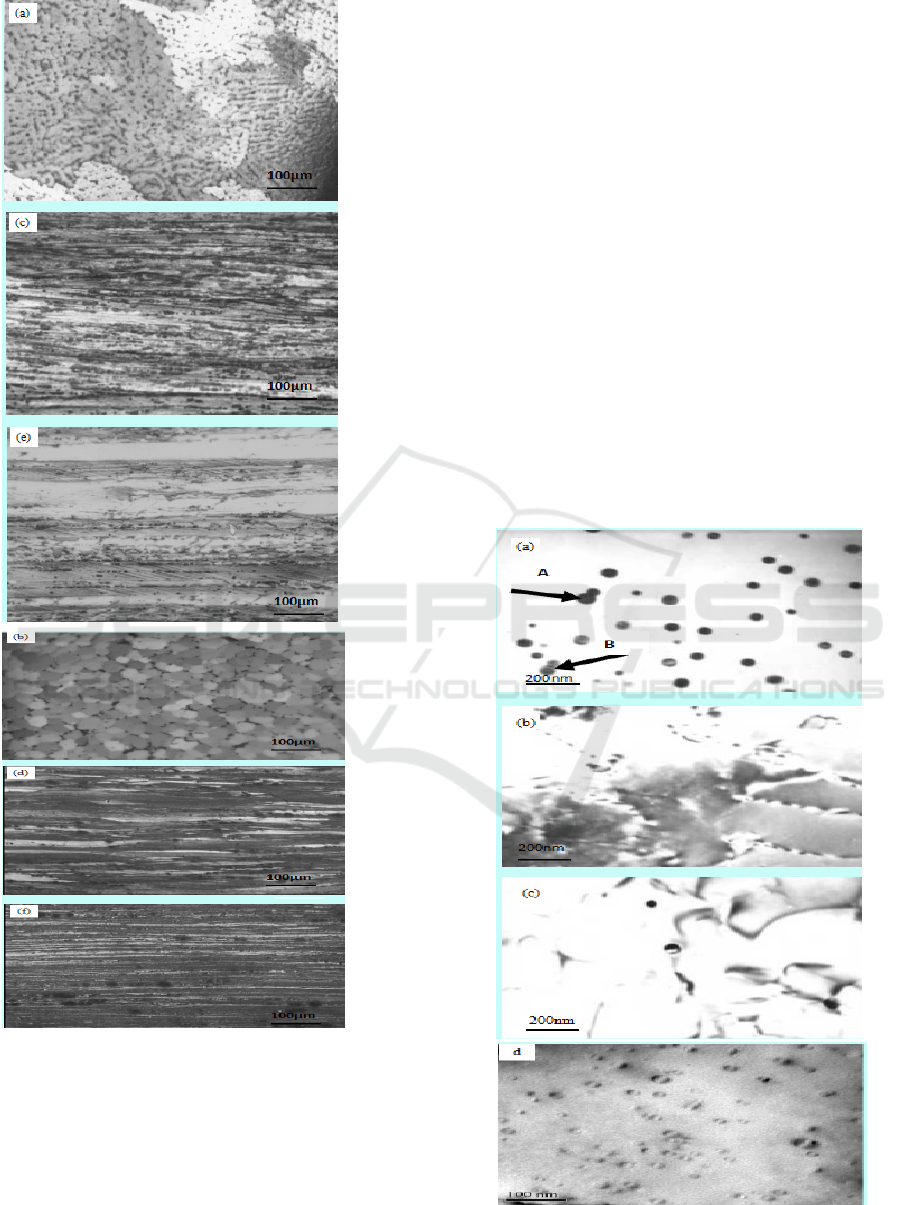
Figure 1: Optical microstructures of the two studied
alloys(a)as-cast organization of alloy A; (b) as-cast
organization of alloy B; (C) hot-rolled organization of
alloy A; (d) hot-rolled organization of alloy B; (e)
annealing organization of alloy A at 340℃/1h; (f)
annealing organization of alloy B at 340℃/1h.
Alloys, thus eliminating the dendritic structure in
them, whereas the grain size in alloy A is above
100μm, in alloy B only 20μm about. So the grain
refinement effect of 0.25%Sc is not obvious, but
trace Zr in Al-Mg-Mn-Sc alloy grain refinement has
a huge role.
Microstructures of the hot rolled alloys are
shown in Figure.1c-d, and Figure.1e-f is that of the
stabilizing annealing processing alloy at 340℃ for
1h. Under these state the 2 alloys possess a fibrous
structure along the rolling direction, never respond
in recrystallization phenomenon. Due to the grain
size in as-cast alloy B is much smaller than that in
alloy A. Therefore, the fibrous structure in alloy B is
much more delicate than that in alloy A. Dense
fibrous structure tensile strength and yield strength
will be higher, which is consistent with the above
the tensile properties. So trace Zr can improve the
organization form of alloy.
3.3 TEM Observation of the Two Studied
Alloys

Figure 2: TEM micrographs of 340℃/1h annealed
alloys(a)The fine, spherical and dispersive secondary
Al
3
Sc particles in alloy A; (b-c) The secondary Al
3
Sc
particles pinning up dislocations and subgrain boundaries
in alloy A; (d) The fine, spherical and dispersive
secondary Al
3
(Sc,Zr) particles in alloy B; (e-f) The
secondary Al
3
(Sc,Zr) particles pinning up dislocations and
subgrain boundaries in alloy A.
Figure 2 shows the TEM microstructures of two
alloys, A and B, both annealed at 340°C for 1h. It is
seen that a large fraction of fine and dispersive
particles have precipitated within grains in these
alloy. These particles are Al3Sc(Fig2.a) in Al-Mg-
Mn-Sc alloy, and Al3(Sc,Zr)(Fig2.d)in Al-Mg-Mn-
Sc-Zr alloy, and they tightly pin up the dislocations
and subgrain boundaries(Figure2.b,c,e,f), and
stabilize the substructure in deformation process.
The gathering and coarsening phenomenon of Al3Sc
particles is observed in fig2.a; as indicated by the
arrows A and B, some particles have intersected and
integrated. Based on the comparison of secondary
phase particles in two kinds of alloys, it could be
found that after adding Zr, the particle size of
Al
3
(Sc,Zr) is smaller than Al
3
Sc, Al
3
(Sc,Zr) particles
are more of dispersion and uniform, and the number
was larger. Thus the role of pinning dislocation and
stabilizing substructure is more intense. In alloy B
the intersected and integrated phenomenon of
Al
3
(Sc,Zr) particles cannot’ be found, and the
substructure also haven’t grown up with smaller size
and a larger number of them. Therefore, Zr in Al-
Mg-Mn-Sc alloy can enhance the effect of
precipitation of second phase particles, retarding the
growth and merging of the secondary particle and
substructure more strongly.
4 DISCUSSION
4.1 Role of Zr in Refining the Grain
Structure of As-cast Alloy
Aluminum alloy as-cast grain size is determined by
the nature of the material itself, the cooling rate and
undercooling degree in the process of solidification,
heterogeneous nucleation core, and so on. Under the
same circumstance, the melt provides more
heterogeneous nucleation core with the grain of
smaller size. According to above experimental
results, compared with Al-5.8Mg-0.4Mn alloy[7],
the as-cast microstructure can’t reach the refinement
effect significantly after adding 0.25%Sc, and the
grain size is above 100μm. This illustrates that the
number of heterogeneous nucleation core in the
process of solidification doesn't get substantial
improvement. Analyzing the phase diagram of Al-
Sc[12] alloy can know that the equilibrium solubility
of Sc is 0.32%. When Sc content is less than 0.25%,
the primary Al
3
Sc particles formed in the
solidification process is not too much, so the
refinement effect to grain is not significant. And
most of Sc in the alloy is more inclined to form a
non-equilibrium supersaturated solid solution which
is unstable and is easy to precipitate in later
processing.
Figure 3: the phase diagram of Al-Sc-Zr at 600℃ and
550℃.

Figure 3[13] is Al-Sc-Zr isothermal phase
diagram of rich aluminum angle at 600℃(solid line)
and 550℃(dotted line). At 600℃ the solubility of Sc
and Zr are 0.09% and 0.06% respectively; at 550℃
are 0.06% and 0.03% respectively. Therefore, Zr on
the one hand, greatly reduces the Sc equilibrium
solubility in Al; on the other hand, it also as a
replacement atom replaces the positions of Sc atom
in Al3Sc. This makes Al
3
Sc particles change into
Al
3
(Sc,Zr) particles, and let Sc atoms have more
chance to form more secondary particle, which gives
the melt to provides a much larger number of
heterogeneous nucleation core, thus refining grain
size significantly. Dependent on the experimental
result, by adding 0.1%Zr in Al-5.8Mg-0.4Mn-
0.25Sc alloy, the grain size reduces from 100μm to
20μm, and the effect is very good.
4.2 Role of Zr to Improve the Mechanical
Properties
From the above analysis, Zr can reduce the
equilibrium solubility of Sc in Al, and can also
improve the degree of supersaturation of the original
solid solution. The increase in degree of
supersaturation makes more and more diffuse
secondary phase Al
3
(Sc,Zr) particles precipitate in
the process of deformation processing and heat
treatment, making pin dislocation, boundary and
sub-structure more intense, so that the strength of the
alloy and recrystallization temperature have greatly
improved.
Zr will also reduce the coarsening rate of
secondary Al
3
(Sc,Zr) particles. Al
3
Sc particles under
the action of heat have tended to gather, grow up and
dissolve back in matrix. Once the Al3Sc particles
grow up, they will lose the coherence with Al matrix,
and also let the distance between the particles
increase, thus losing the role to pin dislocation and
sub-structure, which reduces the mechanical
properties of these alloys. Ye Yicong[14] had
studied the precipitation and coarsening of
secondary Al
3
Sc phase in Al-0.4Sc alloy and found
when aging temperature was greater than 400℃
Al
3
Sc particles grew up quickly, and at 500℃ aging
four hours, these particles had lost coherent
relationship(If particle radius is larger than 20nm, it
will lose coherent relationship). Christian B.
Fuller[15] did the creep experiment at 300℃ for
above one week, and found the Al
3
(Sc,Zr) particles
without apparent coarsening. Figure 4 was
HRTEM(high-resolution electron microscopies)
image of Al
3
(Sc,Zr) precipitates. The preparation of
TEM sample was to use cold-rolled sheet of Al-
5.8Mg-0.4Mn-0.25Sc-0.1Zr alloy to anneal at 550
C for 40h. In the left top corner it was FFT
transformation of white box field in the image. It
illustrates that Al
3
(Sc,Zr) particles haven’t grown up
and are still less than 10 nm in good coherent
relationship with matrix. When 50%Sc atoms in
Al
3
Sc is replaced by Zr atoms, Al
3
Sc phase changes
into Al
3
(Sc
0.5
Zr
0.5
) phase which has the smallest
aggregation bias[11]. The secondary Al
3
(Sc,Zr)
particles have good thermal stability and always
maintain good coherent relationship with matrix, so
strengthening effect is significant.
Fig.4 HRTEM image of Al3(Sc,Zr) precipitates.
5 CONCLUSIONS
(1) Adding 0.1%Zr in Al-5.8Mg-0.4Mn-0.25Sc
alloy can refine the as-cast microstructure
significantly, and the grain size reduces from 100μm
to about 20μm. This is mainly due to the reason that
after adding Zr it can precipitate more primary
Al
3
(Sc,Zr) particles in the casting process, and can
provide more heterogeneous core so as to form more
grains.
(2) The comprehensive mechanical properties
can be improved when 0.1%Zr is added into Al-
5.8Mg-0.4Mn-0.25Sc alloy. Tensile strength(σ
b
) can
be improved by about 20%; the yield strength(σ
0.2
)
can be increased by 30%, and maintain good
elongation. The reasons are that in stabilizing
annealing process more diffuse secondary Al
3
(Sc,Zr)
particles precipitate and the quantity is larger, and
that their thermal stability is better than Al
3
Sc
particles. So the role to pin the dislocation and sub-
structure is more intense, and strengthening effect is
better.

ACKNOWLEDEMENTS
This project was supported by the post doctoral
foundation of Central South University(126226),
the national key basic research development
plan(2012CB619503),the Guangdong Province
Science and technology (2012B011000038) and
the Zhaoqing city science and technology
plan(2013C011).
REFERENCES
1. YIN Zhi-min, ZHU Da- peng , JIANG Feng.
Recrystallization of Al-Mg-Mn and Al-Mg-Mn-Sc-Zr
Alloys[J]. Journal of Materials Engineering,
2004,(6):3-6.
2. WANG Xudong, LIN Shuangping, YANG Junjun,etal.
Microstructure and mechanical properties of Al-Mg-
Mn alloy with erbium[J]. RARE METALS, 2012,
31(3):237-243.
3. Bi-Yu Tang, Dong-Lin Li, Ping Chen, etal. The
thermal properties of Al–Mg–TM (TM¼Sc, Zr):Ab
initiostudy[J]. Solid State Sciences, 2010, (10):845-
850.
4. G.R. Argade, N. Kumar, R.S. Mishra. Stress corrosion
cracking susceptibility of ultrafine grained Al–Mg–Sc
alloy[J]. Materials Science & Engineering A,
2013,565:80-89.
5. HE Zhen-bo, PENG Yong-yi, YIN Zhi-min, etal.
Comparison of FSW and TIG welded joints in Al-Mg-
Mn-Sc-Zr alloy plates[J]. Transactions of Nonferrous
Metals Society of China,. 2011, (21): 1685-1691.
6. Ying WANG, Qing-lin PAN, Yan-fang SONG, etal.
Recrystallization of Al-5.8Mg-Mn-Sc-Zr alloy[J].
Transactions of Nonferrous Metals Society of China,,
2013, 23(11):3235-3241.
7. CHEN Xianming, PAN Qinglin, LUO Chengping, etal.
Effects of micro-alloying with Sc and Ti on the
microstructure and mechanical property of Al-Mg
based alloys[J]. Chinese Journal of Materials Research,
2005, 19(4):419-425.
8. YANG Fubao, LIU Enke, XU Jun, etal. Effects of Er
on the microstructure and mechanical properties of as-
cast Al-Mg-Mn-Zn-Sc-Zr(Ti) filler metals[J]. Acta
Metallurgica Sinica, 2008, 44(8):911-916.
9. Yang Dongxia, Li X Y, He D Y, etal. Microstructure
and Microhardness of Laser Beam Welded Al-Mg-
Mn-Zr-Er Joint[J]. Rare Metal Materials and
Engineering, 2011,40(4):111-114.
10. LIN Shuang-ping,NIE Zuo-ren,HUANG Hui,etal.
Thermodynamic calculation of Er-X and Al-Er-X
compounds existing in Al-Mg-Mn-Zr-Er alloy[J].
Trans. Nonferrous Met. Soc., 2010, (4):682-687.
11. Y. Harada, D.C. Dunand. Thermal expansion of Al3Sc
and Al3(Sc0.75X0.25)[J]. Scripta Materialia,
2003,48:219-222.
12. K. A. Gschneidner Jr., F. W. Calderwood. The Al-
Sc(Aluminum-Scandium) system[J].
Bulletin of Alloy Phase Diagrams, 1989, 10(1):34-36.
13. ZENG Fan hao,XIA Changqing, GU Yi. An
assessment of Al-Mg-Sc-Zr system in aluminum-rich
region[J]. Materials Review, 2002, 16(6):16-19.
14. Ye Yicong, Li Peijie, He Liangju, etal. Precipitation
and coarsening behavior of secondary Al3Sc phase in
Al-Sc binary alloy[J]. Special Casting & Nonferrous
Alloys, 2012,32(3):197-202.
15. Christian B. Fuller, David N. Seidman, David C.
Dunand.Mechanical properties of Al(Sc,Zr) alloys at
ambient and elevated temperatures[J],Acta
Materialia,2003,51:4803-4814.
