
Experimental Study on the Early Immunological Rejection in
Xenotrans
p
lantation of Rabbit Fracture Hematoma Cells
Yan Fang
1
, Xia Dong
1
, Zhengjin Liu
2
and Xiaoping Ying
1
1.
Laboratory of Molecular Pathology, Shaanxi University of Chinese Medicine,
(
Xianyang 712046
)
;
2.
Zhongshan Hospital Xiamen University,
(
Xiamen 361004
)
.
Keywords: Xenotrans plantation, hematoma cells, rejection.
Abstract: Objective: To investigate if xenotransplantation of rabbit fracture hematoma cells in the broken ends of
fractured bone can lead to obvious hyper acute and acute immune rejection. Methods: We conducted a trial
involving 90 rabbits with 4~6 weeks old, weighting 2.2~2.6kg, male and female in half. They were
randomly divided into three groups: fracture group (n=30), fracture transplantation normal saline group
(n=30), and fracture transplantation hematoma group (n=30).3 days after animal models of sawed-off
cubital bone was established, rabbits fracture hematoma cells which were derived from the ordinary livor
blue rabbits’ fracture hematoma and cultured in vitro were transplanted into the broken ends of fractured
bone. Ten rabbits were sacrificed in each group at the 1st, 4th, and 8th day after the transplantation.
Immunohisto chemical method was adopted to observe the survival rate of fracture common chinchilla
rabbit fracture hematoma cells, CD68+ macrophage infiltration and splenic lymph follicles. Immunohisto
chemical SABC method was used to detect the expression of CD68 in the macrophages of New Zealand
white rabbits around the allograft. Results: After transplantation, the fracture hematoma cells of xenogeneic
rabbits survived a lot at the fracture end, and the cells’ structure was normal. The splenic lymphoid follicles
did not proliferate significantly in each group and there was no significant difference in the expression of
CD68 macrophages at each interval between groups. Conclusion: There was no significant immunological
rejection in the early stage of xenotransplantation of rabbit fracture hematoma cells in the broken ends of
fracturedbone.
1 INTRODUCTION
It is well known that the formation of the hematoma
after fracture can promote fracture healing. and it
has also been reported that there are cases and
experiments in fracture treatment with hematoma
[1,2] and these results implied that after
transplantation of the fracture hematoma cells, the
subjects showed early callus formation, large
amount callus formation, and the average fracture
healing time was significantly shorter, indicating
that the fracture hematoma had a significant effect in
fracture healing. However, these studies are only
applicable to autologous hematoma cells, while
limited sources of autologous hematoma cells
cultured in vitro proliferation and poor factors limits
its practical application in the clinic. Therefore,
heterologous transplantation of hematoma cells
could be the better choice. Although the fracture
hematoma cells are in a primitive state with weak
auto antigenicity, there are no reports about whether
the heterogeneous hematomas cell transplantation
will lead to significant immune rejection. Fracture
hematoma cells of heterologous rabbits will be
transplanted into the fracture model in this
experiment to observe the early immune rejection,
and explore the osteogenic potential of heterologous
hematoma cell transplantation in fracture healing.
2 MATERIALS AND METHODS
2.1 Materials
2.1.1 Animals and Groups
The experimental animals were New Zealand white
rabbits bought from the experimental animal center
of The Fourth Military Medical University. We
conducted an experiment involving 90 rabbits with
4~6 weeks old, weighting 2.2~2.6kg, male and
female in half. They were randomly divided into

three groups: fracture group (n=30), fracture
transplantation normal saline group (n=30), and
fracture transplantation hematoma group (n=30).
2.1.2 Cell Culture Reagents and Antibodies
DMEM medium (American Hyclone company),,
which include 100IU/ml penicillin, 100 g/ml
streptomycin (Shanghai Biological Engineering
Technology Co Ltd), 15% newborn bovine serum
(PAA); BrdU and anti BrdU antibody, Goat anti-
rabbit CD68, IgM and IgG first antibody, Rabbit
anti-goat second antibody (Fujian Maixin biological
Technology Development Company).
2.1.3 Main Instruments
OLYMPUS IX70-SIF2 inverted microscope, table
model high speed centrifuge (BIOFUGE STRATOS,
Heraeus company).
3 METHODS
3.1 Isolation and Culture of Fracture
Hematoma Cells
Three days after the model of common livor blue
rabbit femoral fracture, the rabbits were anesthetized
with 3% pentobarbital sodium (30mg/kg) through
ear vein. Hematoma cells from fracture sites were
extracted under aseptic condition, and were put into
Heparin Sodium Single-use Automatic Quantitative
Tube for Blood Specimen Collection (purchased
from Wuhan Zhiyuan Medical Technology Co., Ltd)
, 3ml each. The specimen was shaken up repeatedly
to avoid the formation of small clots, moved to
super-clean worktable and added with 2ml DMEM.
After consecutive pumps with No.4 syringe needle,
it was made into single cell suspension. Following
centrifuge(1000r/min, 10min) to get rid of fat and
supernatant, the remaining cell components were
inoculated into 50ml culture bottle with DMEM,
2ml each. After 7 days under standard environment
(37C°, saturated humidity,5% CO2), the culture
medium was totally replaced and the suspending
hemopoietic stem cells as well as unattached cells
were removed. Then culture medium was replaced
every 3~4 days, and cell shape and growth state
were observed daily through inversion microscope.
Subculture: when a complete layer formed, the cells
were rinsed three times with PBS (purchased from
Hyclone) with the supernatant removed and were
treated with 0.25% trypsinase (from Gibco) and
0.02% EDTA (from Gibco) for 5 min at the ratio of
1∶2 before subculture.
3.2 BrdU Labeling of Fracture
Hematoma Cells
When formed a complete single layer, these 2nd
generation hematoma cells were incubated with
BrdU (terminal concentration:10 mmol/L) for 24h,
followed by washing with non-serum DMEM
medium for 5 times, and were treated with 0.125%
trypsinase and 0.01% EDTA before they were made
into 1×108/ml cell suspension for cell
transplantation [3-4].
3.3 Rabbit Fracture Model
With ulna exposure, the New Zealand rabbits were
conventionally anesthetized, fixed under aseptic
conditions, Ulnas were sawed cross-sectionally
across the middle part with sterile hacksaw blade,
then muscle and skin were sutured. Skin was
disinfected and bound with gauze. 1~5 days after
operation, rabbits were administered penicillin
intramuscularly every day (0.3million unit/kg)
3.4 Transplantation of Fracture
Hematoma Cells
Hematoma cells were transplanted on the 3rd day of
fracture modelling[5]. Group for fracture
transplantation hematoma cell: BrdU marked
hematoma cells were injected with needles
perpendicular to ulna and fracture cross-sections,
with 10mm and13mm in depth, inoculated with 5μL
of cell suspension, and then withdrawn needle
slowly after 10min. Saline group of fracture
transplantation: The physiological saline was
injected according to the above method.
3.5 Test Results
3.5.1 The Growth of Hematoma Cells
After heterologous transplantation, 10 rabbits from
each group were executed at the 1st, 4th and 8th
days, respectively. Their ulnas were taken out and
decalcificated for 60d with 15% neutral
EDTA(purchased from Gibco), continued with
dehydration in a graded series of alcohol before they
were made into paraffin-embedded sections. DAB
staining with anti-BrdU monoclonal antibody ABC
method witnessed that BrdU positive reactants
located at nucleus were brown, granular-like or

distributed diffusedly. 5 sections from each New
Zealand white rabbit were selected and observed
under low-magnification microscope (×10) to count
the total number of Brdu positive cells for statistical
processing.
3.5.2 The Infiltration of Neutrophils at the
Broken End of the Fracture on the
First Day After Xenotransplantation
Was Observed Under Light
Microscope
5 pieces of the ulna paraffin sections of New
Zealand white rabbits executed on the first day after
operation were selected and stained with
conventional HE. 10 slices of vision were taken
from each slice, and neutrophils were counted at
high power microscope (×40). Then the neutrophils
were counted and processed statistically.
3.5.3 Immunohistochemical S-P Assay Was
Used to Detect CD68 Positive
Mononuclear Macrophages at
Fractured End
Immunohistochemistry S-P kits and CD68, produced
in Zgmed, were purchased from Fujian Maixin Bio-
tech Co.Ltd. CD68 positive sections were chosen as
positive control, while PBS, in place of first-
antibody, was taken as negative control. Positive
reaction arose when the total cell count of brown
granules was more than 10%, and scoring was done
based on positive cell count percentage and coloring
intensity, and the sum of the two indexes were
analyzed statistical.
3.5.4 Count Lymphoid Cells
On the 1st, 4th and 8th day after heterologous
transplantation, 10 rabbits from each group were
executed. Their ulnas and spleens were taken out
and rinsed with saline, fixed with 4% neutral
formaldehyde solution and dehydrated in a graded
series of alcohol before they were made into
paraffin-embedded sections. After routine HE
staining, the largest section was observed from five
randomly selected views under four-fold objective
lenses. Meanwhile, lymphoid follicle count was
recorded (when lymphoid follicle covered the
middle-line, the left and upward sites rather than the
right and downward sites were recorded). Then the
data were analyzed statistically andχ2 test was
adopted.
3.6 Statistical Methods
All the data were analyzed with SPSS13.0 software.
Measurement data was expressed with mean
±standard deviation(
−
x
±s), and χ2 test was adopted.
4 RESULTS
1.The 3th day of primary culture (Fig. 1), it can be
seen sporadic hematoma cells, and most of them are
short spindle or triangle. The 12th day of Primary
culture (Figure 2), hematoma cells were fused into
monolayer and primary growth was completed.
Fig. 1: the 3
th
day of primary culture (×100).
Fig. 2: the 3th day of primary culture (×100).
2. Growth status of hematoma cells at different
times in Hematoma Cells group: On the 1st, 4th and
8th days after heterologous transplantation, many
BrdU positive hematoma cells with normal structure
were visible in the transplanted region, and no
obvious degeneration and necrosis could be found.
Positive cell count results showed that according to
comparison of positive cell counts in different post-
operational periods(
−
x
±s), BrdU positive cell count
on the 4th day(57.20 ±4.632) outnumbered that
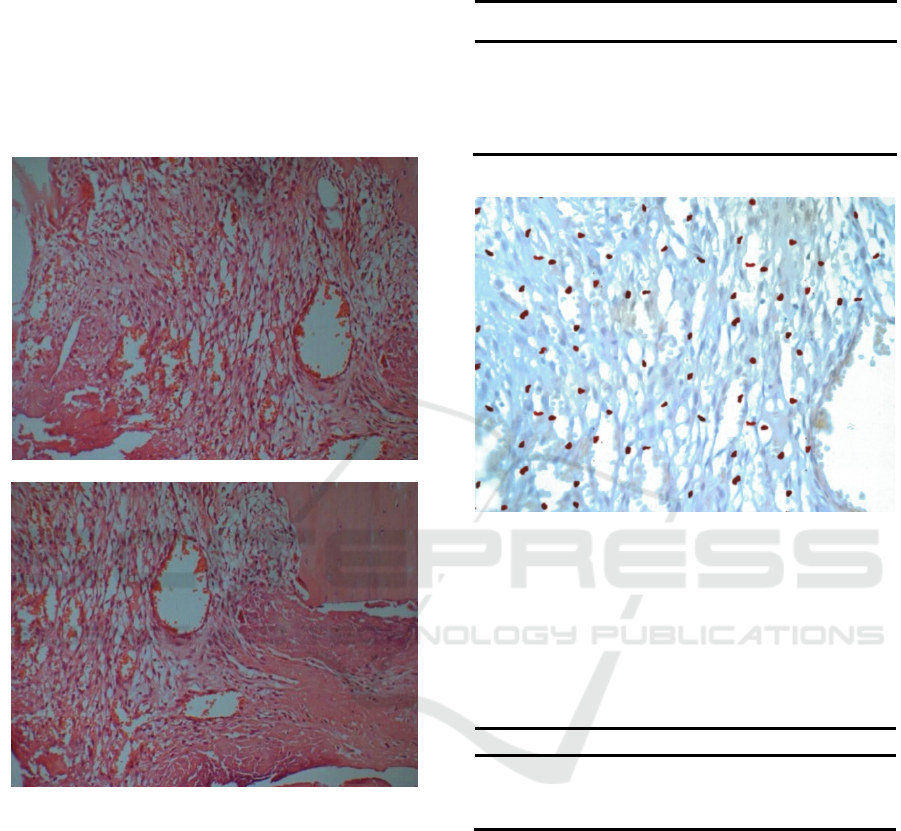
of on the 1st day(38.72 ±5.217) and 8th day(
46.43 ±4.345) (P<0.05).
3.One day after xenotransplantation in New
Zealand white rabbits, neutrophils counts in the
broken ends of fracture bone (
−
x
±s): fracture group
(8.6 + 0.72), saline group (9 + 0.55) and hematoma
cell group (8.9 + 0.43), neutrophils counts have no
significant difference in each group (P > 0.05)
(Figure 3).
Figure 3: Neutrophilic granulocyte infiltration around the
broken end of the fracture (1
th
day) (×100).
4. The expression of IgM and IgG around the
broken end of the fracture bone in New Zealand
white rabbits: no obvious brown granules.
5. Different number of CD68 positive
mononuclear macrophages was found in both the
hematoma group and the control group (Figure 4).
(Table 2)
Table 2: CD68 positive cells counts of the three rabbits’
groups at different time(
−
x
±s).
N Fracture group Saline group Hematoma cell group
1th day 30 0.8±1.1 0.9±0.5 0.9±0.7
4th day 30 4.6±1.2 4.7±1.0 5.0±0.8
8th day 30 5.0±0.7 5.2±0.8 5.3±0.6
Note: no significant difference between each group on the
1th day, 4th day and 8th day after operation (P > 0.05).
Figure 4: Count of lymphoid follicles in the spleen (4th
day) (×200).
6.The count of lymphoid follicles in the spleen
(Table 1)
Table 1: lymphoid follicles counts of three groups of
rabbits after transplantation at different time(
−
x
±s).
Fracture group Saline group Hematoma cell group
1st day 17.6±0.52 18.0±0.55 17.9±0.43
4th day 17.5±0.46 17.7±0.55 18.2±0.50
8th day 18.0±0.34 18.2±0.43 18.4±0.35
Note: no significant difference between each group on the
1th day, 4th day and 8th day after operation (P > 0.05).
5 DISCUSSION
It is known that the hematoma formed by fracture
plays an important role in the process of fracture
healing. The studies on extracting hematoma cells to
promote fracture healing are also confirmed that
hematoma cells have a significant role in fracture
healing [2], however, duo to the limited sources of
cell and the time difference of in vitro hematoma
culture, the practical application chances of it are
greatly reduced. The xenogeneic fracture hematoma

cells are widely distributed, which can be stored in
advance, and also highly proliferative and
multipotential, so they can be one of the best seed
cells to replace autologous hematoma cells
transplantation for the treatment of fracture healing
[6]
. In recent years, the proliferation of in vitro cell
culture is increasingly mature, which makes it
possible to proliferate a large number of primary
cells in the short term and can be reserved for a long
time. In theory, it is considered that the fracture
hematoma cell is a relatively primitive cell with
weak antigenicity. Therefore, allograft
transplantation may cause mild or even no immune
rejection. But up to now, no specific experimental
study at home and abroad was reported.
Schuurman et al. [7] divided xenograft rejection
in the early stage into three categories: hyperacute
rejection (HAR), acute humoral xenograft rejection
(AHXR), and acute cellular xenograft rejection
(ACXR). Studies have shown that the graft non-
function were mainly due to the hyperacute rejection
and the acute dissimilar rejection of the body fluid.
Hyperacute rejection is a leading cause which occurs
within 24 hours after transplantation, It is mainly the
antibody mediated mechanism, which is the humoral
immune response caused by the natural antibody
IgM, and the natural antibody IgG also plays a
certain role[8-10]. Dehoux [11] suggests that anti
IgM and IgG play an important role in activating
endothelial cells and complement. Especially the
induction of anti -Gal IgG is significantly elevated in
AHXR, which may play a major role. The diagnostic
criteria for antibody - mediated acute graft rejection
include 3 basic characteristics [12]: (1)
morphological evidence for acute tissue injury. (2)
immunological evidence of antibody action. (3)
Serological evidence of circulating donor specific
human leukocyte antigen (HLA) antibody or other
donor epithelial cells antigen specific antibodies.
One of the characteristics of AHXR is the
infiltration of all kinds of cells to the grafts. The
existence of neutrophils has a certain predictability
in the diagnosis of AR, and it may represent early
immune response is activated [13-15].
Fischbeck[16] study shows that DXR is mediated by
immune cells such as mononuclear cells.
Mononuclear phagocyte system responsible for
recognition and rejection of xenogeneic antigen in
xenotransplantation [17]; The lymphoid follicles in
the spleen increases when the antigen and blood
circulation enters the spleen and causes humoral
immune response [18]. The results showed that after
the ordinary rabbits hematoma cells were
transplanted into New Zealand rabbits fracture of 1
days、 4 days and 8 days after transplantation, a
large number of xenohematomas survived in the
transplanted region, no obvious degeneration and
necrosis were found and no obvious IgM and IgG
deposition was found around the broken end of the
fracture. There was no significant difference in the
infiltration of neutrophils and CD68 positive
macrophages in the fracture area between each
group in different time. At the same time, there was
no significant difference in the number of splenic
lymphoid follicles in and between groups at different
time, and no significant proliferation of the splenic
lymphoid follicles was found. All these indicate that
there is no obvious rejection reaction between
transplanted rabbit xenogeneic hematoma cells and
their receptors in early stage, and good
histocompatibility also imply that allogeneic
hematoma cells transplantation is feasible, which
provides an experimental basis for future treatment
of fractures or bone defects.
REFERENCES
1. Mizuno K,Mineo K,Tachibana T,et al.The
osteogenetic potential of fracture
haematoma.Subperiosteal and intramuscular
transplantation of the haematoma[J].J Bone Joint
Surg(Br) 1990,72(5):822-9.
2. Guo Hongwang,Li Yuxue. Reutilization of
hematoma at fracture site [J]. China Journal of
Orthopaedics and Tyaumatology,2001,14(1):50-51.
3. Guo Q, Liu Z, Zhanqing L I. Technical explore on
mesenchymal stem cells in bone marrow using BrdU
labelling[J]. Journal of North China Coal Medical
College, 2004,6(1):4-7.
4. Guo Qicang,Zhang Yu,Wang Yufang.
Characteristics and feasibility of bone marrow-
derived mesenchymal stem cells labeled with 5-
bromodexyuridine[J].Chinese Journal of Clinical
Rehabilitation,2006,10(5):144-146.
5. Li Guitao, Li Huaren, Xu Hongzhang. Experiment
Study of the Osteogenetic Potential of Bone
Hematoma[J].Chinese Journal of Bone and Joint
Injury , 2004,19(5):323-324.
6. Grundnes O, Reikers O. The importance of the
hematoma for fracture healing in rats[J]. Acta
Orthopaedica Scandinavica, 1993, 64(3):340-2.
7. SchuurmanHJ,PinoCG,PhllipsMJ,et al.Incidence of
hyperacute rejection In pig-to primate
transplantation using organs from hDAF-transgenic
donors.Transplantation,2002,73(6),1146-1157
8. Koren E, Neethling F A, Richards S, et al. Binding
and specificity of major immunoglobulin classes of
preformed human anti-pig heart antibodies[J].
Transplant International, 1993, 6(6):351.

9. Kujundzic M, Koren E, Neethling F A, et al.
Variability of anti‐αGal antibodies in human serum
and their relation to serum cytotoxicity against pig
cells[J]. Xenotransplantation, 1994, 1(1):58-65.
10. Bach F H. Discordant xenografting: a summary and
look to the future[J]. Transplantation Proceedings,
1992, 24(2):739-742.
11. Dehoux JP,Parra B,Latinne D,et al.Characterization
of baboon anti-porcine IgG antibodies during acute
vascular rejection of porcine kidneyxenograft[J].
Xenotransplantation,2002,9(5):338-349.
12. Zhang Qixu,Zhou Gang. Research progress of
hyperacute rejection in xenotransplantation[J].
Chinese Joumal of Plastic Surgery,2006, 22(6):468-
471.
13. TakahashiH, Kato T, Selvaggi G, et a.l Subclinical
rejection inthe initial postoperative period in small
intestinal transplantation:a negative influence on
graft surviva[J].l Transplantation, 2007, 84(6): 689-
696.
14. Ruiz P, Takahashi H, Delacruz V, et a.l International
gradingscheme foracute cellular rejection in small-
bowel transplantation:single-center experience[J].
TransplantProc, 2010, 42(1): 47-53.
15. Tong W, Bond G,Martin D, et a.l Histopathologic
characteristicsofhuman intestine allograft acute
rejection in patients pretreatedwith thymoglobulin or
alemtuzumab[J]. Am J Gastroentero,l 2006,101(7):
1617-1624.
16. Fischbeck JA,Baier JM,Akella R,et al.Genetic
modification of alphaGalexpression in xenogeneic
endothelial cells yields a complex
immunologicalresponse[J].Tissu Eng ,
2001,7(6):743-756.
17. Papadimitriou J M, Ashman R B. Macrophages:
current views on their differentiation, structure, and
function.[J]. Ultrastructural Pathology, 1989,
13(4):343-372.
18. Lingzhong Cheng. Modern Histology[M].Shanghai
Science and Literature Press, 2003..653-654
