
Growth Method Research of LYSO: Ce Single Crystal
Y P Cai, W P Zhou
*
, X G Bi and J Tang
School of Energy and Power, Shenyang Institute of Engineering, Shenyang,
Liaoning,110136,China
Corresponding author and e-mail: W P Zhou, zhouwp@sie.edu.cn
Abstract. In this paper, growth problems of LYSO: Ce Single Crystal by Czochralski method
are discussed such as crucible erosion, components volatilization, the production of episome
,inclusion and not uniform components and light output. The suggestion of using flame fusion
method to grow the crystal and how to avoid the above problems are put forward.
1. Introduction
With the rapid development of nuclear medicine, high-energy physics, nuclear technology and oil
exploration, the application of inorganic scintillation crystals has been expanded continuously. The
development of scintillation crystal material, the core element of the detection, is increasing, and its
performance is also higher. The scintillation crystals with high density, high light output and fast
delay time can increase the cut-off energy of the ray, improve the threshold of ray detection, and
improve the detection energy resolution (~10%) and time resolution effectively. Cerium-doped
lutetium yttrium oxyorthosilicate single crystal has been the most valuable material [1-4] in nuclear
medical imaging and high energy physics experiments because of its advantages (density 7.10g/cm3,
light output about ~30000ph/MeV, delay time ~40ns).
At present, LYSO:Ce single crystals can be obtained by Czochralski method and optical floating
zone method[4-7]. Only the Czochralski technique can be used for commercial applications. The
Czochralski technique is a basic method of crystal growth. Many but not all.of the crystals used at
present are grown by this method. Compared with the growth of crystals by flame fusion method, the
typical characteristic of the Czochralski method is to use crucible with melt. Therefore, the basic
requirement of crystal growth is that the crucible itself does not oxidize under the condition of
growth, and there is no chemical reaction between the material melt and the crucible material, and the
composition of the melt is kept uniform. The crucible materials often used are graphite, tungsten and
its alloys, platinum and iridium etc. In order to ensure the chemical stability of the crucible under the
conditions of growth, the growth process is generally in the vacuum and inert protection atmosphere.
Obviously, not all materials can meet the above conditions. Such as rutile single crystal, the melt of
its component breaks down under the condition of insufficient vacuum or oxygen partial pressure[8].
For another example, strontium titanate crystals have different melting points in the strontium oxide
component and the titanium oxide component in the raw material powder, which will change the
composition of the melt in the state of the melt.
Cai, Y., Zhou, W., Bi, X. and Tang, J.
Growth Method Research of Lyso: Ce Single Crystal.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 685-689
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
685

2. Problems and reasons for growth of Ce: LYSO single crystal by Czochralski method
The first problem in the growth of Ce:LYSO crystals by Czochralski method is the melting of
crucible. Ce:LYSO crystal is a high temperature oxide crystal. The crystal itself and its components
have very high melting point, see Table 1, so they can not grow in vacuum or reducing atmosphere.
A metal iridium crucible is used in the growth of the crystal. At such a high temperature, even if
there is no oxygen in the growing environment, the oxygen molecules dissociation will be made from
Lu
2
O
3
due to the intensification of the molecular heat movement in production. In order to restrain
oxygen molecules dissociating to the environment, the oxygen partial pressure in the atmosphere is
often added, which is bound to add to the atmosphere. The iridium gold crucible is oxidized. In this
case, the iridium crucible is particularly volatile or melted into the melt, which not only increases the
growth cost, but also makes iridium gold particles easy to enter into the crystal and form the light
scattering center, such as the growth process of cerium doped lutetium silicate single
crystal(Ce:Lu
2
SiO
5
) by Czochralski method [9].
Table 1. The physical and chemical properties of Ce: LYSO raw materials and
various components.
raw materials and
their composition
Ce :LYSO SiO
2
Y
2
O
3
Lu
2
O
3
CeO
2
density(g/cm
3
) 7.3-7.4 2.2 5.01 9.42 7.65
melting point(°C) 2050-2070 1710 2410
2467-
2510
2400-
2600
boiling point(°C) - 2230 4300 3980 3500
The second problem is that in the composition of Ce:LYSO, the melting points differ greatly
among SiO
2
, Lu
2
O
3
, Y
2
O
3
and CeO
2
, as shown in Table 1. This leads to the following problems, fist
is that during the calcination process, Lu
2
O
3
, CeO
2
and Y
2
O
3
with high melting point are not easily
diffused to form a single phase of Ce:LYSO structure, and the free Lu
2
O
3
, CeO
2
and Y
2
O
3
particles
may exist in the formed Ce:LYSO matrix structure; next is that the components of Lu
2
O
3
, CeO
2
and
Ce:LYSO with high melting point may not melt yet in the formed melt, but remain in the melt in
granular form. In these two cases, the solid phase inclusions are produced in the generated crystals;
the last is that the SiO
2
components with low melting point are easy to volatilize, which results in the
change of the composition of the melt and the difficulty in the growth of high quality crystals.
The third problem is the density difference among SiO
2
, Lu
2
O
3
, CeO
2
and Y
2
O
3
. See Table 1.The
SiO
2
components with smaller density will float above the melt, which makes the composition of the
melts different in the vertical direction. So the crystal composition is not uniform, even no crystal can
grown.
The fourth problem is that due to the small segregation coefficient of Ce, about 0.20-0.25, the
bottom concentration of Ce ions in the growing LYSO crystal is far higher than the top. This uneven
distribution makes the bottom light output higher than the top light output, and the attenuation time
will be increased accordingly, so only a part of the grown crystal is available. At the same time, the
energy resolution becomes worse.
These factors have led to the technological complexity and crystal properties instability of
Ce:LYSO single crystal grown by Czochralski method. Because of the use of the crucible, the
corrosion of the crucible resulted in the contamination of the crystal and the increase of the growth
cost,. This problem can be avoided, if the crystal is grown by the flame fusion method which does
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
686

not use the crucible, In the compositions of Ce:LYSO, although the melting point among SiO
2
, Y
2
O
3
,
Lu
2
O
3
and CeO
2
is very different, the damage will also be greatly reduced, if the low melting point
components are not volatile in the growing environment and the time of the melt state is short. In the
same way, although the density difference among SiO
2
, Lu
2
O
3
and CeO
2
and Y
2
O
3
is very large, the
degree of component delamination will be greatly reduced if the crystal is grown directly after the
raw material is melted rather than in the melt state for a long time. Although shortening the melt
holding time can not eliminate the effect of too small segregation coefficient, increasing the growth
speed can still reduce its effect.
3. Foundation analysis of the growth of Ce: LYSO single crystal by flame fusion method
In view of the problems above existing in the Czochralski growth method of Ce: LYSO single
crystal, the author proposes to grow Ce:LYSO single crystal by flame fusion method. At present, this
method has not been reported to grown the Ce:LYSO single crystal. Compared with Czochralski
growth method, the flame fusion method is also a method of growing the single crystal from the melt.
It is different that the flame fusion method does not need the melt crucible when the crystal is grown.
At the same time, the melt droplet drops directly on the seed crystal or the growing crystal after
melting through the high temperature hydrogen oxygen flame, and the melt keeps time very short.
Therefore, the use of flame fusion method to grow Ce:LYSO crystals will avoid the problems
encountered by the Czochralski method.
There is no need for crucible in the growth of crystals by flame fusion method, and the crystal
growth process can be carried out in an oxidizing or reducing atmosphere according to the melt
properties of the grown crystal. There is no problem of iridium crucible corrosion when Ce:LYSO
single crystal is grown.
The growth of crystals by flame fusion method is often based on the hydrogen and oxygen flames,
such as corundum, rutile and strontium titanate. The temperature of the hydrogen and oxygen flame
center can reach 2800°C, and the temperature distribution of the flame is gradually reduced from the
highest temperature center to around, the burner and the growth chamber are designed reasonably,
the position of the growth interface is controlled well, and the raw materials and the components of
the Ce:LYSO are normal at the same time. As the partial pressure of oxygen can be controlled in the
growth environment, and the melt holding time is very short, as shown in Figure 1, the possibility of
the loss of the SiO
2
component with low melting point is greatly reduced, eliminating the problems
of the solid phase inclusions in the crystals of high melting point components, Y
2
O
3
, Lu
2
O
3
and
CeO
2
.
Component layering due to the different density of each component in the melt in the Ce:LYSO
single crystal grown by flame fusion method can be well inhibited, as shown in Figure 1. The degree
of component layering is directly proportional to the height of melt and the duration of melt state.
The height of the melt is h, the density of the low density component of the SiO
2
melt is ρ, the
floating velocity is v and the component is uniform when time is t0 . Then the distribution of the low
density component SiO
2
along the Y direction of the melt is:
)tt(v
y
C
01
SiO
2
−=
∂
∂
ρ
hy ≤≤0
(1)
Growth Method Research of Lyso: Ce Single Crystal
687
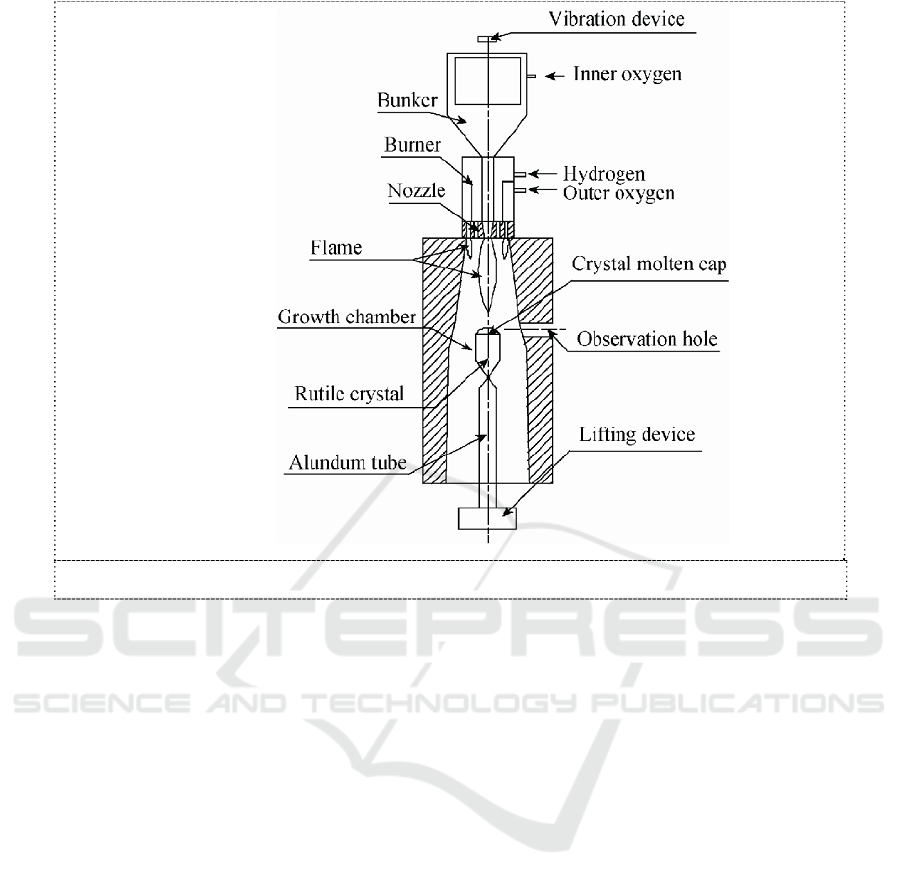
Figure 1. Figure of crystal growth by way of flame method
The growth rate of the flame fusion method is generally about 10mm/h, but the speed of the
growth of the crystal is generally at 0.5-1.0mm/h by Czochralski growth method,. We can see from
the formula (1) that the degree of the melt delamination is more than 10 times of the flame fusion
method. Therefore, the growth of Ce:LYSO single crystal by the flame fusion method can effectively
restrain stratification of the different density components in the melt.
The growth rate of Ce:LYSO single crystal from melt by Czochralski growth method is about 10
times slower than that by flame fusion method. It can be seen as about 10 times the speed of the
growth interface (solid liquid interface) advancing to the melt. Because the segregation coefficient of
Ce is between 0.22-0.25, that is, the effective segregation coefficient is less than 1. When other
factors (boundary layer thickness and so on) remain unchanged, the effective segregation coefficient
increases with the acceleration of the solid liquid interface[10], which reduces the uneven distribution
of Ce in the Ce:LYSO single crystal, and effectively solves the instability of the crystal properties
caused by the Ce segregation of the Ce:LYSO single crystal grown by Czochralski growth method.
4. Conclusions
This paper give the suggestion of using flame fusion method to grow Ce:LYSO single crystal which
can avoid the problems such as crucible erosion, components volatilization, the production of
episome ,inclusion and not uniform components and light output.
Acknowledgement
The authors would like to thanks for Shenyang Sunrise Crystal Technology Co., Ltd. for its strong
support in the experiment
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
688

References
[1] Zhang M R and Wei J 2004 status of research and development on scintillationcrystals with
Properties of high density and fast decay time J. CHINESE CERAMIC SOCIETY 32(3)
384-391
[2] Pidol L, Kahn-Harari A, Viana B and et al 2004 Scintillation detectors high efficiency of
lutetium silicate scintillators, Ce-doped LPS,and LYSO J. IEEE Trans Nucl Scl.51 (3)
1084
[3] Kimble T, Chou M, Chai B H T and et al 2002 Scintillation proper-ties of Ce: LYSO crystals J.
IEEE Nuclear Sci. SympConf. 3 1434
[4] Chen J M, Zhang L Y, Zhu R Y 2005 Large sizeLYSO crystals for future high energy physics
experiments J. IEEE Trans Nucl Scl. 52 3133
[5] Chen J M, Mao R H and Zhang Y 2007 Large size LYSO-LSO crystals for future high energy
physics experiments J. IEEE Trans Nucl Scl. 54 718
[6] Lecoq P 1994 The high energy physics demand for a new generation of scintillators J. J
Lumin 60-61 948 -955
[7] Blahuta S, Bessière A, Viana B and et al Defects Identification and Effects of Annealing on
Lu2(1-x) Y2xSiO5 (LYSO) Single Crystals for Scintillation Application
[8] Bi X G, Liu X D and Niu W 2012 Flame-fusion Growth of Rutile Single Crystal J. Advanced
Engineering Materials II 5 535-537
[9] Wang J, Cen W, Li H X, Jiang C J and Shi Z B 2013 Growth of Ce: LYSO Scintillation Crystal
with Large Size, Piezoelectrics & Acoustooptics 36(3) 7 401-407
[10] Zhang K C and Zhang L H 1997 Crystal growth science and technology M. 3rd
ed.vol.1,Beijing:Science Press 412-413. 83-91
Growth Method Research of Lyso: Ce Single Crystal
689
