
Impedance Spectra Characteristics of Dy-doped Fe-20Cr
Alloys in the Presence of Solid K
2
SO
4
-KCl Mixture at 600°C
in Air
Y B Lai, P Y Guo*, Y Shao, H Sun, J T Ding, Y X Wan, Y X Qiao and Y X Guo
School of Materials Science and Engineering, Jiangsu University of Science and
Technology, Jiangsu 212003, China
Corresponding author and e-mail: P Y Guo, pyguo@just.edu.cn
Abstract. Impedance spectra characteristics of Fe-20Cr, Fe-20Cr-0.2Dy and Fe-20Cr-1Dy
alloys are studied in 0.5K
2
SO
4
-0.5KCl mixture in air at 600°C. The two-electrode system is
used for electrochemical impedance measurements, and it has been proved effective in the
study of solid- and molten-salt corrosion. The corrosion of alloys goes through two stages
during extended exposure. Impedance spectra of every stage is fitted by an equivalent circuit
and corresponding electrochemical elements. The effect of Cr content in Fe-based alloys has
been investigated in an attempt to understand corrosion mechanism involving the
active/oxidation process of metal Cr. The effect of the additive Dy dissolving and
precipitating in Fe-Cr alloy on the hot corrosion has also been investigated. Corrosion
products in different stages are analyzed by scanning electron microscopy and the formation
and depletion of protective scale in mixed salt is monitored. Corrosion rate and corrosion
mechanism of three alloys are discussed.
1. Introduction
KCl-K
2
SO
4
mixture is able to attack the metal surfaces of the superheaters and leads to serious
corrosion problems. The development of a material that has a high-corrosion resistance in the
combustion deposit containing chloride and sulfate has been advanced[1-3]. High-Cr ferritic and
austenitic steels are widely used as high-temperature components and protective chromia scales
formed on these alloys surfaces make an immediate impact in corrosion resistance during
high-temperature exposure.
In order to improve alloys against high temperature corrosion and extend service life of alloys,
some reactive elements can be added in alloys. The rare earth effect is made up of a number of
different factors for high temperature corrosion[4-7], such as selective oxidation enhanced, the
predominantly transport mechanism altered, void formation suppressed and the adherence of scales
increased. The corrosion behavior of Ni-Dy alloys[8,9] showed that a small amount of Dy could
retard greatly the corrosion of Ni and the incorporation of Dy into nickel oxide decreased clearly its
solubility in molten (Li,K)
2
CO
3
mixture at 650°C. The oxidation of Co-Y and Fe-Y binary alloys at
600-800°C in air[10,11] showed that alloys produced an external scale and a wide internal oxidation
region. The rate of oxygen penetration in Co-Y and Fe-Y alloys was faster than in pure cobalt and
iron, but outward diffusion cobalt and iron decreased. Oxidation behavior of Dy-doped Fe-20Cr
674
Lai, Y., Guo, P., Shao, Y., Sun, H., Ding, J., Wan, Y., Qiao, Y. and Guo, Y.
Impedance Spectra Characteristics of Dy-doped Fe-20Cr Alloys in the Presence of Solid K2SO4-KCl Mixture at 600°C in Air.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 674-680
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

(wt.%) alloys at 900°C in air[12] showed that Dy could significantly improve the oxide resistance of
Fe-20Cr. Although a few papers were published on the oxidation behavior of Dy modified alloys, the
experiments performed in the solid, partially melted and completely melted KCl-K
2
SO
4
mixture for
the long exposure time were still interesting.
In this study the high temperature corrosion behavior of Fe-20Cr alloys with the addition of Dy is
investigated using simultaneous electrochemical impedance techniques. Test-cell design is important
to electrochemical measurements for corrosion of materials. Zeng[13,14] discloses that a
two-electrode arrangement comprising two working electrodes can be prepared conveniently, and is
also suitable for simple electrochemical impedance measurements.
2. Experimental
Fe-20Cr, Fe-20Cr-0.2Dy and Fe-20Cr-1Dy (wt.%) are employed in the present study. Back-scattered
electron images of the annealed alloys are shown in Figure 1. A few white particles are found in
Fe-20Cr-0.2Dy, which are Dy-rich precipitates and most are localized at grain boundaries. The
solubility of Dy is more lower than 1 wt.% in Fe-20Cr alloys. Fe-20Cr-1Dy are two-phase composed
of a Fe solid solution and a Dy-rich phase. Alloy ingots are cut into pieces in the size of
10mm×5mm×1 mm.
The EIS measurements are conducted in the mixed 0.5K
2
SO
4
-0.5KCl salt (mole fraction) in air at
600°C. The mixed salt is dried at 400°C for 48h and then heated to reaction temperature. During the
experiment specimens are totally buried under the mixed salt for about 25mm, and extracted at
selected intervals for morphology analysis with the residual potassium salts. A two-electrode system
is used for EIS measurements (Figure 1c). The Fe–Cr wire is spot-welded to one end of the specimen
for electric connection. Two specimens parallel to each other and are sealed in an alumina tube by a
high-temperature cement, with one side uncovered. The high-temperature cement is solidified at
100°C for 24 h and 350°C for 10 h. The size of each working electrode is 5mm×5mm×1mm and they
are degreased again for test. The distance of two electrodes is about 1mm to reduce electrolyte
resistance between the gap. EIS measurements are performed at open circuit potential between 1×10
-2
and 1×10
4
Hz using a computer-controlling high-speed Parstat 2273 of Princeton Applied Research.
The amplitude of input sine-wave voltage is 10 mV.
Figure 1. Microstructure of (a) Fe-20Cr-0.2Dy and (b) Fe-20Cr-1Dy alloys (bright phase: Dy-rich
phase; dark phase: solid solution), (c) two-electrode system for EIS.
10μ
m
(a)
Dy-rich phase
10μ
m
(b)
Dy-rich
5 mm
5 mm
1 mm
1 mm
Alumina
tube
High-temperature
(c)
Impedance Spectra Characteristics of Dy-doped Fe-20Cr Alloys in the Presence of Solid K2SO4-KCl Mixture at 600°C in Air
675
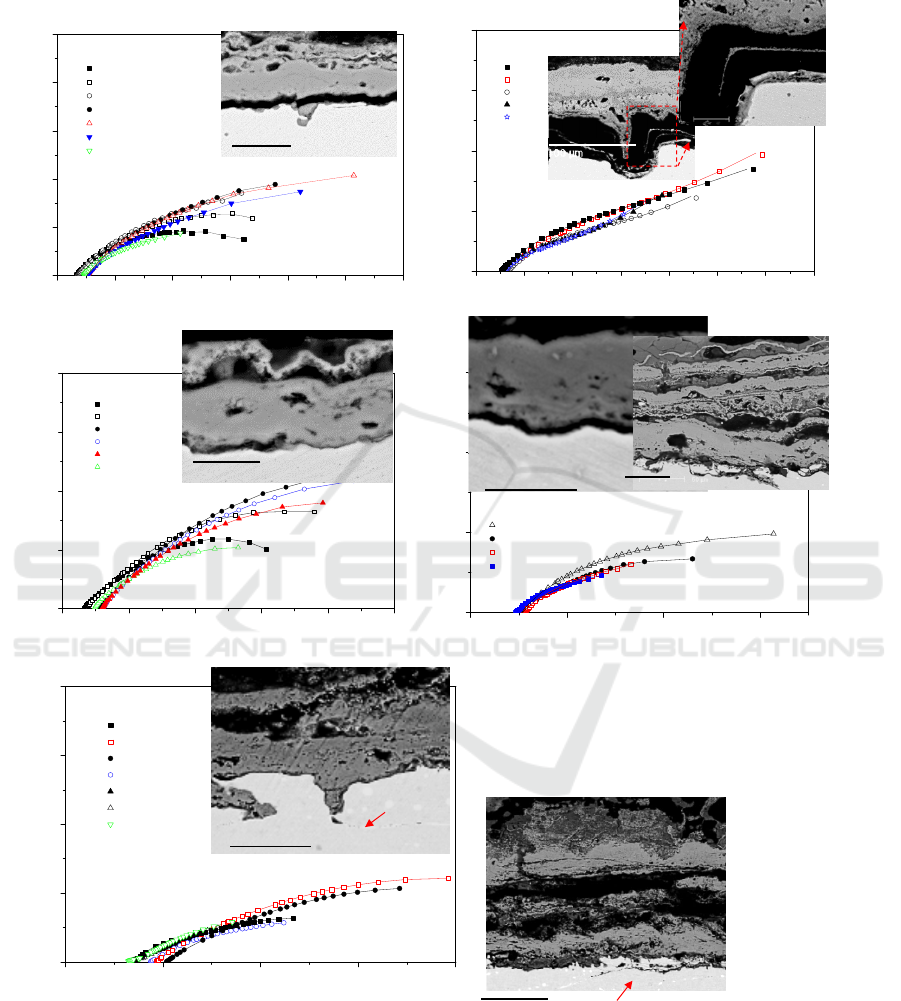
3. Results and discussion
3.1. Impedance spectra and corrosion products in solid mixed salt
0 1000 2000 3000 4000 5000 6000
0
1000
2000
3000
4000
5000
Z
im
, Ω·cm
2
137h
117 h
65 h
8 h
49 h
3 h
1 h
600
o
C Fe-20Cr
1 h
3 h
8 h
49 h
65 h
117 h
137 h
Z
re
, Ω·cm
2
300 600 900 1200 1500 1800 2100 2400
0
500
1000
1500
2000
150h
232h
275h
369h
414
600
0
C Fe-20Cr
Z
re
,
Ω·cm
2
Z
im
,Ω·cm
2
232h
150h
275h
369h
414h
0 1000 2000 3000 4000 5000
0
1000
2000
3000
4000
Z
im
, Ω·cm
2
Z
re
, Ω·cm
2
149h
117 h
93 h
40 h
7 h
600
o
C Fe-20Cr-0.2Dy
1 h
7 h
40 h
93 h
117 h
149 h
1 h
0 1000 2000 3000
0
1000
2000
3000
345h
414h
275h
232h
600
o
C Fe-20Cr-0.2Dy
Z
re
,Ω·cm
2
Z
im
,Ω·cm
2
232h
275h
345h
414h
0 1000 2000 3000 4000
0
1000
2000
3000
4000
Z
im
, Ω·cm
2
Z
re
, Ω·cm
2
60 h
33 h
138h
1 h
1 h
10 h
33 h
60 h
88 h
116 h
138 h
600
o
C Fe-20Cr-1Dy
10 h
Figure 2. Nyquist plots for alloys buried in K
2
SO
4
-KCl mixtures at 600
o
C in air after different
exposure times and cross-sectional morphology of alloys after exposure.
Figure 2 shows EIS and cross section of Fe-20Cr corroded in solid mixed salt at 600°C for different
time. The Nyquist plots show that it is a large capacitive loop at all frequencies between 1h and 137h,
and the diffusion-controlled reaction clearly develops after electrodes corroded for 232h. The
external oxides of Fe-20Cr corroded for 117h and 414h are mainly compact Fe/Cr oxides and some
(a)
(b)
(c)
20μm
10
μ
m
50
μ
m
100μm
20
μ
m
414h
117h
10μm
232h
117h
116h
50
μ
m
100μm
internal sulfidation/oxidation
internal sulfidation/oxidation
414h
414h
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
676
sulfides are found in oxide/metal interface. There is no internal oxidation in Fe-20Cr alloy, but the
alloy interface prominently fluctuates after corrosion for 414h.
The Nyquist plots of Fe-20Cr-0.2Dy are composed of a large capacitive loop at all frequencies
during the period of 345h, and the modulus of impedance |Z| is obviously larger than Fe-20Cr alloy
in initial stage. The Nyquist plots show a line at the low frequency part after corroded for 345h,
which means the degradation of protective scale. Oxide products of Fe-20Cr-0.2Dy are mainly
Cr-rich oxides and it is thin and compact with a good adhesion to alloy for 117h and 232h. The oxide
scale for 414h is composed of an external scale of Fe/Cr oxides plus a mild internal
sulfidation/oxidation. Some residual salt is found near to the outermost oxide layer.
The Nyquist plots of Fe-20Cr-1Dy maintain a large capacitive loop at all frequencies during the
period of 138 h, however, the modulus of impedance is obviously smaller than that of Fe-20Cr and
Fe-20Cr-0.2Dy. Cross section of Fe-20Cr-1Dy shows that the external oxide layer is a little thick. An
internal sulfidation/oxidation is observed along the grain boundary in which Dy-rich precipitates are
localized. With the extended hours, thick external oxide layer becomes loose and some chlorides are
found in the interface of oxide/alloy.
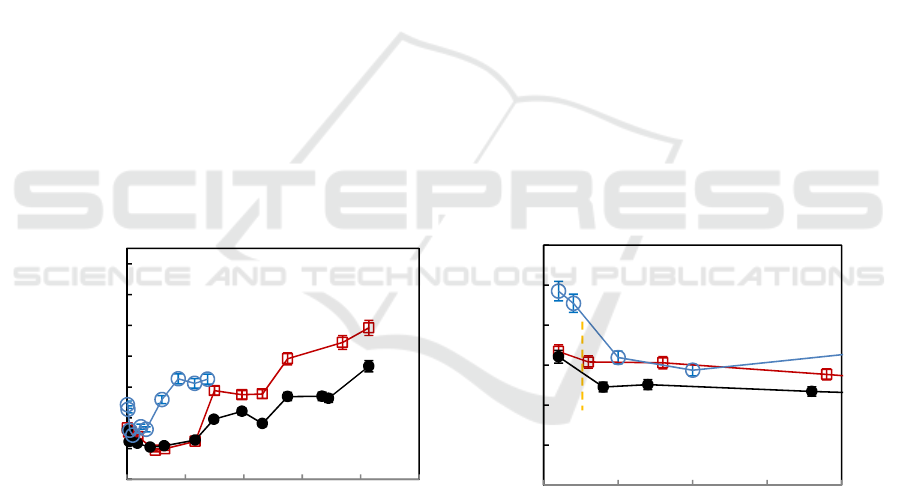
3.2. Corrosion rate
The corrosion rate V
t
is proportional to the reciprocal of polarization resistance and the evolution
with exposure time is shown in Figure 3. Corrosion rates of three alloys increase with the reaction
time. Corrosion rate of Fe-20Cr-1Dy is a little higher than Fe-20Cr and Fe-20Cr-0.2Dy after about
100h. Corrosion rates of Fe-20Cr and Fe-20Cr-0.2Dy in the stage of 100h are almost the same, and
then corrosion rate of Fe-20Cr increases and becomes higher than Fe-20Cr-0.2Dy between 100h and
414h. The magnified view shows that corrosion rate of alloys decrease rapidly to a minimum
corresponding to about 5h, and then rises gradually, which means the formation of protective scales
in the initial stage.
Figure 3. The evolution of corrosion rate with exposure time in mixed salt at three different
temperatures.
3.3. Electrochemical impedance models
When alloys corroded at 600°C, reaction temperatures are lower than the melt point of mixed salt. So
kali salt on the top of alloys is still solid in the initial stage. Electrochemical impedance spectroscopy
is assumed to be only the response of oxide scales forming on the surface. An equivalent circuit of
oxide capacitance parallel with scale resistance can be used to represent the single capacitive loop for
the corrosion (Figure 4a). With reaction time increasing, mixed kali salt between two electrodes is
sintered and may be the semi-melting status. The impedance of a diffusion-controlled reaction can be
represented by the equivalent circuit (Figure 4b).
0
0,0002
0,0004
0,0006
0,0008
0,001
0,0012
0,0014
0 100 200 300 400 500
vt, Ω
-1
·cm
-2
Time, h
Fe-20Cr-0.2Dy
Fe-20Cr
Fe-20Cr-1Dy
600℃
0
0,0001
0,0002
0,0003
0,0004
0,0005
0,0006
0 5 10 15 20
vt, Ω
-1
·cm
-2
Time, h
Fe-20Cr-0.2Dy
Fe-20Cr
Fe-20Cr-1Dy
600℃
Impedance Spectra Characteristics of Dy-doped Fe-20Cr Alloys in the Presence of Solid K2SO4-KCl Mixture at 600°C in Air
677
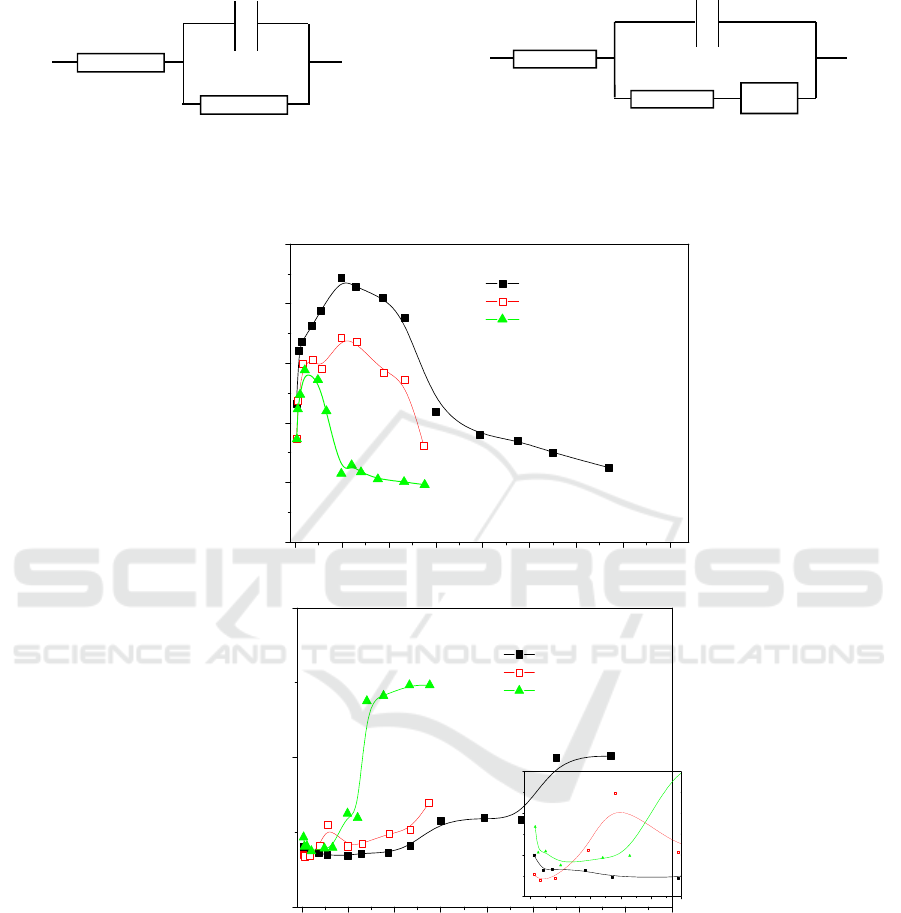
Figure 4. Equivalent circuits for interpretation of EIS.
0 50 100 150 200 250 300 350 400
0.0
2.0x10
3
4.0x10
3
6.0x10
3
8.0x10
3
1.0x10
4
R
ox
, Ω·cm
2
Time, h
Fe-20Cr-0.2Dy
Fe-20Cr
Fe-20Cr-1Dy
600
o
C
0 50 100 150 200 250 300 350 400
0.000
0.001
0.002
Fe-20Cr-0.2Dy
Fe-20Cr
Fe-20Cr-1Dy
Y
ox
, Ω·cm
-2
·S
-n
Time, h
600
o
C
0 1020304050
0.0003
0.0004
0.0005
0.0006
Figure 5. Changes of R
ox
(a) and Y
ox
(b) with exposure time in K
2
SO
4
-KCl mixture at 600°C.
In the presence of the solid K
2
SO
4
-KCl mixture, three alloys undergo a two-stage corrosion
behavior during the period of 414 h. In the incubation period, the corrosion of alloys is mainly
associated with the formation of some oxides, because the salt between electrodes is in the solid state.
In the second period, the double-layer capacitance arises in reaction process. Fitting results of
impedance spectra discloses that the incubation period for Fe-20Cr is about 150 h, Fe-20Cr-0.2Dy
about 345 h, and Fe-20Cr-1Dy more than 138h. Transfer resistance of ions
ox
R
increases at the
beginning and rapidly reaches a peak value, and then decreases to an appropriate range (Figure 5a).
(a)
R
s
C
ox
R
ox
(b)
R
s
C
dl
R
t
Z
w
(a)
(b)
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
678

The values of
ox
R
for Fe-20Cr-0.2Dy are the greatest in three alloys.
ox
R
of Fe-20Cr-1Dy is
roughly coincident with Fe-20Cr during the period of 24h. However, the values sharply decline after
33h. The results indicate that the protection of oxide scales formed on Fe-20Cr-0.2Dy is better than
Fe-20Cr, but that on Fe-20Cr-1Dy is worse than Fe-20Cr, especially for the long exposure duration.
The values of oxide capacitance
ox
Y in Figure 5b show that
ox
Y gradually increases with time.
The values of Fe-20Cr are the larger than other alloys and that of Fe-20Cr-0.2Dy is the smallest. The
evolution of
ox
Y is consistent with transfer resistance of ions through the scale.
3.4. Corrosion mechanism
The corrosion of alloys involves complicated reaction stages including the incubation period and
acceleration period. At the initial stage, oxidation occurs at the salt/alloy interface.The increasing of
Cr content in Fe-based alloys is beneficial to the formation of protective oxides. Furthermore, the
proper addition of Dy could also contribute to the formation of protective oxide scale. In the next
reaction process, the K
2
SO
4
-KCl mixture will readily react with the Fe oxides, Cr oxides and Dy
oxides in the oxide/salt interface. At the same time, reactions in the oxide/alloys interface may also
occur due to the penetration of K
2
SO
4
and KCl through the macro-cracks or pores within the oxide
scales. The large amount of chlorine forming will evidently increase chlorine partial pressure and
decrease oxygen partial pressure. The gaseous chlorine gradually diffuses into oxide/alloy interface,
and directly reacts with alloys to form the solid FeCl
2
, CrCl
2
and DyCl
2
, respectively. When the
partial pressure of chloride is high enough, the gaseous chloride diffuses back into the oxide/salt
interface
[14]
. Fe oxides, Cr oxides and Dy oxides again precipitate on the zone of high oxygen partial
pressure. The oxide scale formed by this way is rather porous and can hardly provide any effective
protection, so that the corrosion rate is significantly enhanced. The active/oxidation process results
show that the external oxide layer is an expanded and layered structure especially for alloys corroded
for a long time (Figure 2). Also, the thickness of external oxide layer remarkably increases by this
way.
Furthermore, the sulphate is commonly hard to diffuse through the compact oxides. When the
oxide scale is destroyed by chloride, sulphate diffuses into oxide/alloy interface through cracks or
pores of the oxide scales and reacts with alloy. Equilibrium partial pressures of solid Cr chlorides are
much lower than Fe chlorides to produce the solid Cr
2
O
3
. The test results show that corrosion
resistance of Fe-20Cr-1Dy is poor in K
2
SO
4
-KCl mixture and the external oxide scales are
particularly loose. It is assumed that equilibrium partial pressure of solid Dy chloride is lower than
that of Fe/Cr chlorides or the addition of Dy accelerates the volatilization and re-deposition of Fe/Cr
chlorides. When Dy-rich phase precipitates at the grain boundary of Fe-Cr alloy, the intergranular
oxidation becomes prominent and the penetration depth of internal oxidation front increases.
4. Conclusions
EIS has been utilized to synchronously monitor the corrosion of Fe-20Cr, Fe-20Cr-0.2Dy and
Fe-20Cr-1Dy alloys in 0.5K
2
SO
4
-0.5KCl mixture at 600°C. Corrosion rates of alloys increase with
increasing temperature and rise fast with mixed salt partially melting down. Electrochemical
parameters calculated from the equivalent circuit discover transfer resistance of ions and charge
transfer resistance of Fe-20Cr-0.2Dy are the greatest and followed by Fe-20Cr and Fe-20Cr-1Dy
among the three alloys. The detrimental effect of chromium is showed in Fe-20Cr, Fe-20Cr-0.2Dy
and Fe-20Cr-1Dy alloys, and corresponding porous and layered corrosion products are showed in
cross-section morphology. The addition of Dy accelerates the volatilization and re-deposition of
Fe/Cr chlorides.
Impedance Spectra Characteristics of Dy-doped Fe-20Cr Alloys in the Presence of Solid K2SO4-KCl Mixture at 600°C in Air
679

Acknowledgement
This work was financially supported by National Natural Science Foundation of China (51201073),
China Scholarship Fund (2017), research fund of Jiangsu University of Science and Technology
(1624821607-5), and Postgraduate Research & Practice Innovation Program of Jiangsu Province
(KYCX17_1830).
References
[1] Fukumoto M, Tachikawame C, Matsuzaka Y and Hara M 2012 Corrosion Science 56 105
[2] Shinata Y 1987 Oxidation of Metals 27 315
[3] Guo P Y, Zhang J Q, Young D J and Konrad C H 2015 Oxidation of metals 83 223
[4] He J, Zhang Z, Peng H, Gong S K and Guo H B 2015 Corrosion Science 98 699
[5] Lai Y B, Guo P Y, Shao Y, Zhang Y and Liu N 2017 Journal of Alloys and Compounds 694
383
[6] Brylewski T, Gil A, Rakowska A, Chevalier S, Adamczyk A, Dabek J, Kruk A, Stygar M and
Przybylski K 2013 Oxidation of Metals 80 83
[7] Lan H, Yang Z G, Xia Z X, Zhang Y D and Zhang C 2011 Corrosion Science 53 1476
[8] Zeng C L, Zhang T, Guo P Y and Wu W T 2004 Corrosion Science 46 2183
[9] Liu Z P, Guo P Y and Zeng C L 2007 Journal of Power Sources 166 348
[10] Zeng C L, Rizzo F C, Wu WT and Monteiro M J 1999 Corrosion Science 41 1731
[11] Zeng C L, Rizzo F C, Monteiro M J and Wu W T 1999 Oxidation of Metals 51 495
[12] Guo P Y, Zeng C L, Shao Y and Qin Z S 2012 Journal of Rare Earths 30 1150
[13] Zeng C L and Zhang T 2004 Electrochimica Acta 49 1429
[14] Zeng C L and Li J 2005 Electrochimica Acta 50 5533
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
680
