
Fabrication of Dense Aluminum Nanoparticle Arrays with
Controllable Deep Ultraviolet Surface Plasmon Resonance
Properties
Y Y Ding, G Xu, J A Chen, F Liu and M Han
*
National Laboratory of Solid State Microstructures, Department of Materials Science
and Engineering, and Collaborative Innovation Centre of Advanced Microstructures,
Nanjing University, Nanjing 210093, China
Corresponding author and e-mail: M Han, sjhanmin@nju.edu.cn
Abstract. UV irradiation was used to tailor the surface plasmon band of the densely
distributed Al nanoparticle arrays fabricated by gas phase deposition. We showed that the
broad surface plasmon resonance band of the as-prepared sample could be tuned to a sharp
and strong resonance band in the DUV optical range, with a large blue shift of the peak
wavelength. The evolution of the surface plasmon resonance properties was attributed to the
UV irradiation-improved surface oxidation of the nanoparticles, which vanished the nearfield
couplings between the closely spaced nanoparticles by increasing their interspacing.
1. Introduction
Aluminium nanoparticles (Al NPs) are of interest to a variety of applications, such as photocatalysts
[1], optical coatings [2], transparent conductive films [3], as well as propellant and explosive
materials [4]. They have been receiving considerable interest lately as plasmonic materials alternative
to gold and silver NPs, with their attractive properties such as low cost, high natural abundance, and
advantages in device performance, design flexibility, processing and tenability [5-11]. Al NPs are
especially attractive for UV plasmonics because they exhibit surface plasmon resonance (SPR)
properties in the full UV range. Particularly, the SPR of small Al NPs locates in the deep ultraviolet
(DUV) region of the optical spectrum, which is of great interest in numerous applications; e.g.
ultrasensitive organic molecule sensing [5, 6] and photocatalysis[7,8]. Short wavelength UV light is
capable of breaking organic bonds, which is the key to biological applications.
The surface plasmons (SPs) of Al NPs and their assemblies are extremely sensitive to the
geometrical characteristics [9, 12-14]. The SPR bands can be tuned in a wide spectrum range from
the DUV to the IR by varying the particle size, shape as well as the inter-particle spacing. For an
individual Al NP, the SPR wavelength increases with its diameter D and reaches 300 nm at D=70
nm[9], which means it is difficult to generate SPR at DUV wavelengths with Al NPs fabricated with
standard lithography techniques[15]. For NPs prepared by various bottom-up synthetic methods, the
distribution in particle size and impurities induce broadened or even featureless SPR spectra. The
Ding, Y., Xu, G., Chen, J., Liu, F. and Han, M.
Fabrication of Dense Aluminum Nanoparticle Arrays with Controllable Deep Ultraviolet Surface Plasmon Resonance Properties.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 591-597
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
591

impact of oxidation is huge to the smaller NPs, which are concerned majorly in DUV applications.
Furthermore, aggregation of the NPs in the dense array induces large red-shift and broadening of the
SPR bands due to the nearfield coupling among the closely spaced NPs. Therefore challenges still
remain to synthesize well-controlled Al NPs suitable for DUV plasmonic applications.
As a poor metal, aluminium is easily oxidized when expose to the atmosphere. A thin native oxide
layer can be formed on the surface so that the Al NP is wrapped with a shell. The formation of
alumina layer is an important property. It acts as a passivation layer and prevents further oxidation of
the Al NPs. As a result, highly stable and discrete Al NPs can ultimately be prepared. Furthermore,
the SPR of the Al NPs also depends sensitively on the presence of the dielectric oxide shell [16,17].
Consequently, oxidation can be a means to tune the SPR of the Al NPs by controllable growth of the
dielectric shell. In this paper we report the evolution of the UV SPR bands of the densely distributed
Al NPs induced by surface oxidation assisted by UV light irradiation. We show that the UV
irradiation can sufficiently tune the SP spectra of the gas-phase synthesized Al NP arrays into a sharp
and strong resonance in the DUV optical range.
2. Materials and methods
2.1. Preparation of Al NPs.
We used a magnetron gas aggregation cluster source [18] to generate Al NPs in gas phase. Atoms
were sputtered from the Al target and Al clusters were formed through the aggregation process in the
argon gas. A stable argon gas flow was introduced into the liquid nitrogen cooled aggregation tube to
maintain a constant carrier gas pressure for cluster growth. The cluster size was controlled by the
carrier gas pressure. The clusters were swept by the gas stream into a high vacuum chamber through
a nozzle and deposited on the UV-grade fused silica substrate surface.
2.2. Characterization and optical measurements.
The size and morphology of the Al NPs was characterized with a transmission electron microscope
(TEM). The extinction spectra of the Al NP arrays are collected in a transmission configuration using
a UV-vis spectrophotometer equipped with a deuterium lamp light source. The measurement was
performed at normal incidence.
3. Results and discussion
Figure 1 shows the TEM image of the Al NPs prepared under argon gas pressure of 50 Pa. As shown
in the figure, the Al NPs distributed on the substrate surface randomly and aggregations occurred
among most of the particles. The average diameter of the NPs was measured to be 19 nm, with a size
distribution of about 6 nm.
Figure 2 shows an extinction spectrum of the Al NP arrays collected in a transmission
configuration using a UV-vis spectrophotometer equipped with a deuterium lamp light source. The
measurement was performed at normal incidence. The spectrum is dominated by a very broad
resonance peak, covering the wavelength range from about 210 nm to longer than 400 nm. The peak
wavelength is about 270 nm. The spectrum displayed little change when measured following several
days of atmospheric exposure, implying that the NPs were passivated effectively with the self-
terminating native Al oxide. Also shown in Figure 2 is the extinction coefficient for an individual 14
nm Al sphere encapsulated with a 3 nm oxide shell calculated using the finite difference time domain
(FDTD) method. Significant discrepancies appear when comparing the experimental and calculated
spectrum. The experimental spectrum displays a large red-shift (>60 nm) and becomes very
broadened. Its shape also departures from the characteristic Lorentzian resonance of a dipolar
oscillator.For small Al NPs, the SPR bands redshift with increasing particle diameter, so that the size
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
592
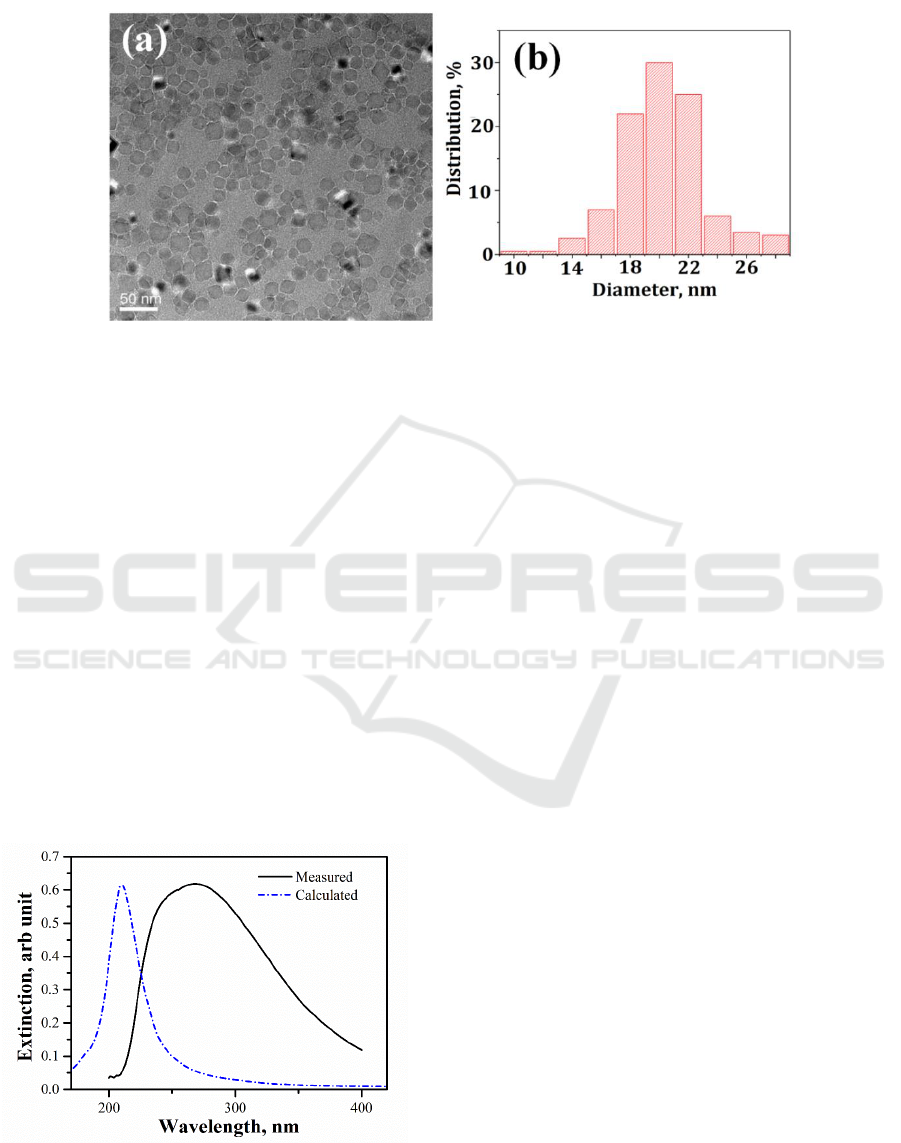
Figure1. (a) TEM image of Al NPs generated with cluster beam deposition. (b) Histogram
measured from the TEM images.
distribution exists in the NP arrays may induce SPR band broadening. However the size distribution-
induced broadening should not dominate the experimental spectrum since only less than 25 nm red-
shift could be expected [19] when the size of the NP changes from 15 nm to 25 nm, a size region
which includes most of the Al NPs. We thus attribute the red-shift and broadening of the
experimental spectrum observed herein to the near field couplings occurr among the closely spaced
NPs in the dense arrays. It has been reported that the near-field couplings between NPs in close
proximity will not only broaden the resonant peak but also red-shift the resonant wavelength [20].
The fractional plasmon red-shift (Δλ/λ, where λ denotes the SPR wavelength and Δλ is the red-shift
induced by near-field coupling) decays near-exponentially over a ratio of inter-particle spacing S/D,
that is [21]: ∆λ/λ~exp(-K·S/D), where K is a constant. This means shorter interparticle spacing
permits much stronger nearfield coupling, which generates a larger red-shift. Previous research
showed [22] that in the dense array of 8 nm sized Ag NPs generated by cluster beam deposition, with
the increase of the NP density the SPR wavelength demonstrated a redshift from less than 400 nm to
more than 570 nm, in accompanying with an increased broadening of the resonance peak. Although
near-field coupling could sufficiently tune the SPR bands in a wide wavelength region, which will be
important for many applications[13,15], for DUV plasmonics the red-shift and broadening of the
SPR band will be failed to satisfy the practical application conditions, especially when a high density
of NPs is required to provide sufficient enhancement.
Figure 2.Experimental extinction spectrum
(solid) of the Al NP arrays, normalized to the
bare fused silica substrate, and the calculated
extinction coefficient (dashed line) of an
individual 14 nm Al NP coated with a 3 nm
oxide shell.
Fabrication of Dense Aluminum Nanoparticle Arrays with Controllable Deep Ultraviolet Surface Plasmon Resonance Properties
593
We found that the SPR band of the Al NP arrays could be tailored by UV light irradiation. To
exam this, the Al NP samples were attached on the rotatable sample stage of the UV-vis
spectrophotometer equipped with a 30 W deuterium lamp light source. Real-time extinction spectra
were collected in a transmission configuration at room temperature every 5 minutes for holding times
up to 140 min. Meanwhile, the NPs were exposed to the UV illumination of the deuterium lamp
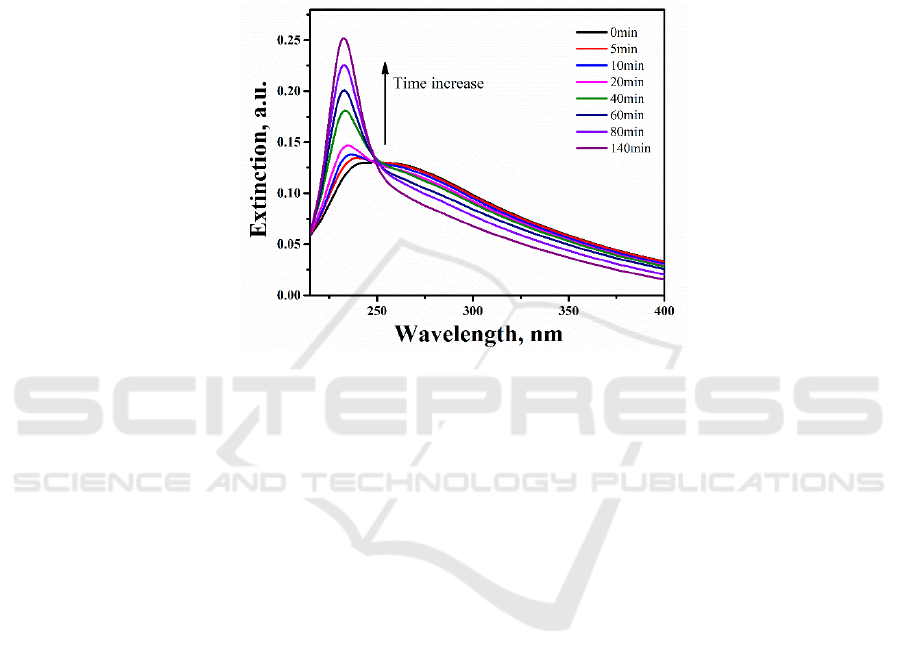
continuously. Shown in Figure 3 are representative extinction spectra recorded during UV irradiation.
Figure 3.Extinction spectra of the Al NP arrays recorded in real time during UV irradiation in air at
room temperature. Total holding time is 140min.
With UV irradiation, a sharp extinction band between 220 and 250 nm rose at the shorter
wavelength edge of the original broad band, in accompanying with a continuous decrease in the
extinction peak intensity at longer wavelength. The new extinction band became more and more
intense with the increase of the UV irradiation time. The new band peaked at 240 nm with 5 min
irradiation, and monotonously shifted to shorter wavelength when increasing the UV exposure time.
Also evident was the continuous narrowing of the extinction band. With 140min UV irradiation, the
original broad SPR band almost vanished, remaining a sharp intense SPR band peaks at 232 nm, with
a full width at half maximum (FWHM) of about 20 nm.
The evolution of the SPR band in Al NP arrays under UV irradiation might be attributed to the
growth of the oxide layers on the NP surfaces inspired by UV light. The change on the oxide shell
thickness of the NPs varied both the size and interspacing of the NPs. In Figure 3, the broad band in
longer wavelength was attributed to the near-field coupled SPs of closely spaced Al NPs, while the
new arisen sharp band in shorter wavelength DUV region could be attributed to the intrinsic SPs of
the isolated Al NPs. UV irradiation induced thicker Al oxide layers, which increased the interspacing
and vanished the nearfield couplings between them. With the increase of the oxide shell thickness,
the proportion of the isolated NPs became larger, which resulted in the pronounced shorter
wavelength SPR band that corresponded to the isolated Al NPs. On the other hand, the SPR
wavelength of an isolated Al NP depended sensitively on the presence of the oxide shell on its
surface. The variation caused by NP oxidation depended on two factors, which generated opposite
changes: the reduction of the metallic core size leaded to a blue shift and narrowing of the SPR band,
whereas an increase of the effective refractive index surrounding the core resulted in a red shift and
broadening of the SPR band. For smaller Al NPs, as the oxide shell increased, the blue-shift and
sharping compensated and dominated the red-shift and broadening [10, 17]. Therefore, the results on
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
594
the time evolution of the SPR for the Al NP arrays under UV irradiation appeared consistent with the
trends predicted with the above analysis, i.e., the rising of the DUV SPR band, and the FWHM
decrease and blue shift of its peak.
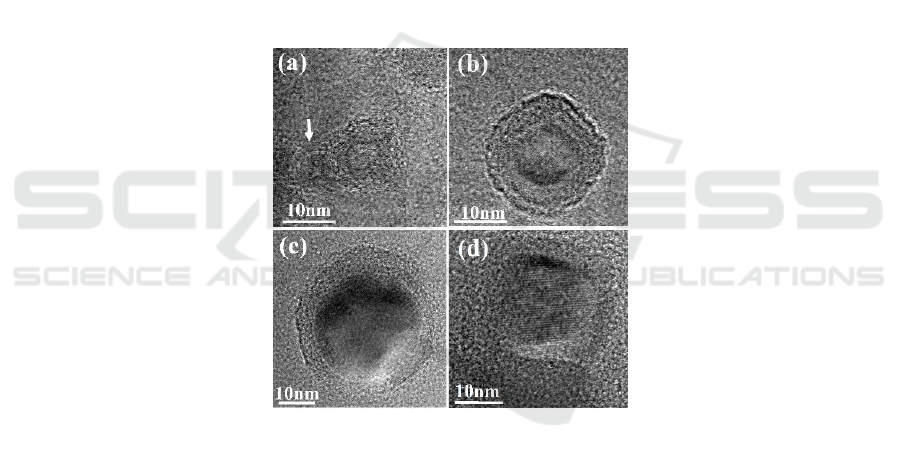
The increase of the oxide shell thickness of the Al NPs under UV irradiation can also be verified
with high resolution TEM (HRTEM). Figure 4(a)-(c) shows HRTEM images of three individual Al
NPs with different diameters (D = 15, 23 and 40 nm). Prior to the HRTEM observation the NPs had
been exposed to UV irradiation in atmospheric ambient for 140min. From these images, spherically
shaped core/shell nanoparticles could be clearly observed. Lattice images were distinguished in the
cores, implying they were Al nanocrystals. We believed that the amorphous shells were most likely
Al
2
O
3
. Although the oxide shell was not very uniform for each individual Al NPs, its thickness kept
around 6 nm on average and did not vary with the NP size. It should be noted that the oxide layer
observed here was considerable thicker than those reported for small Al particles previously
(typically 2.5 nm) [23, 24]. In Figure 4(d), a HRTEM image of an individual Al NP without UV
irradiation is also shown. The sample had been exposed to atmospheric ambient for four days before
the observation. Comparing with the NPs shown in Figure 4(a)-(c), its oxide shell was much thinner
and obscure.
Figure 4.(a)-(c) HRTEM images of individual UV exposed Al NPs with different sizes, (a) D = 15
nm, (b) D=23 nm, (c) D= 40 nm, respectively. (d) HRTEM image of an individual Al NP without
UV exposing.
Figure 5 shows the extinction coefficient of two closely touched 20 nm Al nanoparticles using
FDTD method, where t represents the thickness of the oxide shell on the surface of the nanoparticles.
When t=0, two Al nanoparticles are in contact, resulting in a wide band that extends to over 400nm at
the long wavelength due to near-field coupling. When two nanoparticles have the oxide layer, the
wide extinction band at the long wavelength disappears, while a sharp extinction peak appears at the
wavelength below 250 nm. With the thickening of the oxide layer, the extinction peak further blue
shifts .This calculation is in line with the previous experimental results.
Fabrication of Dense Aluminum Nanoparticle Arrays with Controllable Deep Ultraviolet Surface Plasmon Resonance Properties
595
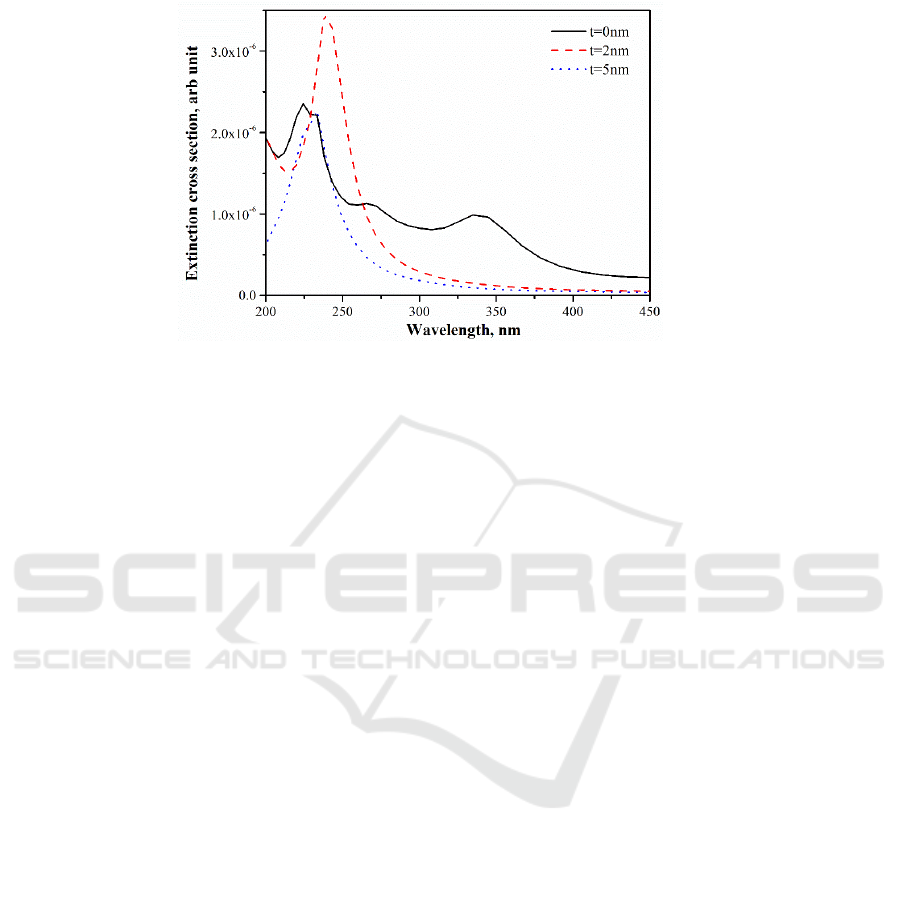
Figure 5.Extinction coefficient of two closely touched 20nm Al nanoparticles with different surface
oxide shell thickness.
4. Conclusions
In summary, we have fabricated dense arrays of Al NPs with an average size of about 19 nm by the
gas phase cluster beam deposition method. The NP arrays exhibited broad SPR spectra in the UV
region, owing to the red-shift induced by the near field couplings between the closely spaced
nanoparticles. UV irradiation have been used to tailor the plasmonic properties in Al NP arrays. We
have shown that the UV irradiation induced a large blue shift of the SPR band, resulted in a sharp and
strong SPR band in the DUV region, peaked at 232 nm with a FWHM of 20nm. The evolution of the
SPR property was attributed to the growth of the oxide shells on the NP surfaces inspired by UV light,
which vanished the near-field couplings between the closely spaced Al NPs by increase their
interspacing. The increase of the oxide shell thickness of the Al NPs under UV irradiation has been
verified by HRTEM. We have demonstrated an easy way to realize intense DUV SPR in stable Al
NP arrays, which might find broad applications, such as ultraviolet Raman spectroscopy, sensing,
and photovoltaics.
Acknowledgments
We thank the financial support from the National Natural Science Foundation of China (Grant nos.
11627806, 11604161, 61301015), the National Basic Research Programme of China (973 Program,
Grant nos. 2014CB932302). This research was also supported by a project funded by the Priority
Academic Programme Development of Jiangsu Higher Education Institutions.
References
[1] Zhou L, Zhang C, McClain M, Manjavacas A, Krauter C, Tian S, Erg F B, Everitt H, Carter E,
Nordlander P and Halas N 2016 Aluminum nanocrystal as a plasmonic photocatalyst for
hydrogen dissociation Nano Lett. 16 1478-84
[2] Lachebi I, Fedala A, Djenizian T, Hadjersi T and Kechouane M 2018 Morphological and
optical properties of aluminum nanoparticles deposited by thermal evaporation on heated
substrates Surf. Coat. Tech. 343 160-5
[3] Lee Y J, Lee C and Lee H M 2018 Synthesis of oxide-free aluminum nanoparticles for
application to conductive film Nanotechnology 29 055602
[4] Lynch P, Fiore G, Krier H and Glumac N 2010 Gas-phase reaction in nanoaluminum
combustion Combust. Sci. Technol. 182 842-57
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
596

[5] Kikawada M, Ono A, Inami W and Kawata Y 2015 Surface plasmon-enhanced fluorescence
cell imaging in deep-UV region Appl. Phys. Express 8 072401
[6] Taguchi A, Hayazawa N, Furusawa K, Ishitobi H and Kawata S 2009 Deep-UV tip-enhanced
Raman scattering J. Raman Spectrosc. 40 1324-30
[7] McClain M, Schlather A, Ringe E, King N, Liu L, A Manjavacas, M Knight, I Kumar, K
Whitmire, H Everitt, P Nordlander and N Halas 2015 Aluminum nanocrystals Nano Lett.
15 2751-5
[8] Honda M, Kumamoto Y, Taguchi A, Saito Y and Kawata S 2014 Plasmon-enhanced UV
photocatalysis Appl. Phys. Lett. 104 061108
[9] Knight M, King N, Liu L, Everitt H, Nordlander P and Halas N 2014 Aluminum for
plasmonics ACS Nano 8 834-40
[10] Maidecchi G, Gonella G, Zaccaria R, Moroni R, Anghinolfi L, Giglia A, Nannarone S, Mattera
L, Dai H, Canepa M and Bisio F 2013 Deep ultraviolet plasmon resonance in Aluminum
nanoparticle arrays ACS Nano 7 5834-41
[11] Knight M, Liu L, Wang Y, Brown L, Mukherjee S, King N, Everitt H, Nordlander P and Halas
N 2012 Aluminum plasmonic nanoantennas Nano Lett. 12 6000-4
[12] Sanz J, Ortiz D, Osa R A, Saiz J, González F, Brown S, Losurdo M, Everitt H and Moreno F
2013 UV plasmonic behavior of various metal nanoparticles in the near- and far-field
regimes: geometry and substrate effects J. Phys. Chem. C 117 19606-15
[13] Tan S, Zhang L, Zhu D, Goh X, Wang Y M, Kumar K, Qiu C and Yang J 2014 Plasmonic
color palettes for photorealistic printing with Aluminum nanostructures Nano Lett. 14
4023-9
[14] Ekinci Y, Solak H and Löffler J 2008 Plasmon resonances of aluminum nanoparticles and
nanorods J. Appl. Phys. 104 083107
[15] Gérard D and Gray S 2015 Aluminium plasmonics J. Phys. D: Appl. Phys. 48 184001
[16] Kuzma A, Weis M, Flickyngerova S, Jakabovic J, Satka A, Dobrocka E, Chlpik J, Cirak J,
Donoval M, Telek P, Uherek F and Donoval D 2012 Influence of surface oxidation on
plasmon resonance in monolayer of gold and silver nanoparticles J. Appl. Phys 112 103531
[17] Gutierrez Y, Ortiz D, Sanz J, Saiz J, Gonzalez F, Everitt H and Moreno F 2016 How an oxide
shell affects the ultraviolet plasmonic behavior of Ga, Mg, and Al nanostructures Opt.
Express 24 20621-31
[18] Han M, Xu C, Zhu D, Yang L, Zhang J, Chen Y, Ding K, Song F and Wang G 2007
Controllable synthesis of two-dimensional metal nanoparticle arrays with oriented size and
number density gradients Adv. Mater. 19 2979
[19] Hu J, Chen L, Lian Z, Cao M, Li H, Sun W, Tong N and Zeng H 2012 Deep-ultraviolet blue-
light surface plasmon resonance of Al and Al-core/Al
2
O
3
shell in spherical and cylindrical
nanostructures J. Phys. Chem. C 116 15584-90
[20] Rechberger W, Hohenau A, Leitner A, Krenn J, Lamprecht B and Aussenegg F 2003 Optical
properties of two interacting gold nanoparticles Opt. Commun. 220 137-41
[21] Su K, Wei Q H, Zhang X, Mock J, Smith D and Schultz S 2003 Interparticle coupling effects
on plasmon resonances of nanogold particles Nano Lett. 3 1087-90
[22] Gong Y, Zhou Y, He L, Xie B, Song F, Han M and Wang G 2013 Systemically tuning the
surface plasmon resonance of high-density silver nanoparticle films Eur. Phys. J. D 67 87
[23] Ramaswamy A and Kaste P 2005 A 'nanovision'' of the physiochemical phenomena occurring
in nanoparticles of aluminum J. Energ. Mater. 23 1-25
[24] Levitas V, McCollum J and Pantoya M 2015 Pre-stressing micron-scale Aluminum core-shell
particles to improve reactivity Sci. Rep. 5 7879
Fabrication of Dense Aluminum Nanoparticle Arrays with Controllable Deep Ultraviolet Surface Plasmon Resonance Properties
597
