
Hierarchical Porous Carbon Materials Derived from
Microwave-assisted Heating of Zeolitic Imidazolate
Frameworks for Use as Supercapacitor Electrodes
J Z Zou
1, 2
, S Y Liu
1
, H L Wu
1,*
, Q Luo
1
, L Huang
1,3
, X R Zeng
1,2
, J Ma
1
, Y C
Yao
1
, Q Zhang
4
and B L Peng
1
1
Shenzhen Key Laboratory of Special Functional Materials & Shenzhen Engineering
Laboratory for Advance Technology of ceramics, College of Materials Science and
Engineering, Shenzhen University, Shenzhen, 518060. P.R. China
2
Guangdong JANUS Intelligent Group Corporation Limited, Dongguan, 441900,
P.R. China
3
Key Laboratory of Optoelectronic Devices and Systems of Ministry of Education
and Guangdong Province, College of Optoelectronic Engineering, Shenzhen
University, Shenzhen 518060, P.R. China
4
School of Aerospace, Transport and Manufacturing, Cranfield University, Cranfield,
Bedfordshire, MK43 0AL, UK
Corresponding author and e-mail: H L Wu, whl@szu.edu.cn
Abstract. A series of hierarchically structured nanoporous carbons are prepared via the direct
carbonization of zeolitic imidazolate frameworks (ZIF-8) using a microwave-assisted heating
method for the first time. The results indicate that the microwave carbonization temperature
has a remarkable influence on the surface area and pore structure of the obtained porous
carbons. ZIF-8 provides the carbon and the microporous structure. Significant numbers of
mesopores and macropores emerge in the samples carbonized at high temperatures, indicating
a clear d ifference from carbons prepared using traditional heating methods. The resulting
porous carbons possess surface areas ranging from 384.4 to 947.5 m
2
g
-1
and pore volumes
ranging from 0.17 to 0.49 cm
3
g
-1
. For a carbonization temperature of 1000 °C , the porous
carbon possesses the largest specific capacitance of 207.7 F g
-1
at 0.1 A g
-1
and the highest
retention of 66.5% when the current density increases from 0.1 to 10 A g
-1
, which should be
attributed to the proper hierarchical pore structure and the relatively high graphitization
degree.
1. Introduction
Due to the rapid consumption of fossil fuel resources and associated environmental problems,
research into efficient energy storage systems has attracted considerable interest. Among the various
energy storage devices, compared to traditional batteries, supercapacitors have attracted more and
more attention due to their high power densities, fast charging and discharging characteristics, long
cycle lives, less environmental pollution, etc. These devices have been widely used in digital devices,
electric vehicles, communication technologies and other fields [1-3]. Research has been focused on
Zou, J., Liu, S., Wu, H., Luo, Q., Huang, L., Zeng, X., Ma, J., Yao, Y., Zhang, Q. and Peng, B.
Hierarchical Porous Carbon Materials Derived from Microwave-assisted Heating of Zeolitic Imidazolate Frameworks for Use as Supercapacitor Electrodes.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 571-583
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
571

increasing the energy density of supercapacitors without weakening the high power density and
stable cyclic life. Currently, nanoporous carbon (NPC) materials are identified as one of the most
promising candidates for supercapacitor electrode materials due to their high thermal and chemical
stabilities, large specific surface areas, controllable pore structures, high conductivities, easy
manufacturing in large quantities with relatively low costs, etc [4-6]. In the electrochemical capative
performance, the pore size distributions of the porous carbons play an important role, and a
hierarchical pore structure with well-interlinked small and large pores is regarded to provide new
accesses to improve both the specific capacitances and rate capabilities of porous carbons as
supercapacitor electrodes [7-12]. As a result, many approaches have been studied to prepare highly
porous carbons and adjust their pore structures, including laser ablation [13], chemical vapour
decomposition (CVD) [14], arc discharging [15] and templating [16,17], as well as physical or
chemical activation methods [18]. Among these methods, the template method has become one of the
most studied approaches for the preparation of nanoporous carbons due to its regulated architecture,
relatively narrow pore size distribution, and desirable physicochemical properties [19]. However, the
templating process requires additional pore-forming templates and acid treatment steps to remove the
templates, which result in enhancement of preparation cost and difficulty of utilization for large-scale
production.
To solve these major problems, a class of novel porous materials, crystalline metal-organic
frameworks (MOFs), have attracted an enormous amount amount of attention because they can act as
excellent precursors or sacrificial templates for the preparation of nanoporous carbons (NPCs) due to
their extremely high specific surface areas, favourable thermal stabilities, tailored pore structures and
adjusted channels [20-23]. Importantly, MOFs can act as both the templates and the carbon sources,
thus reducing the producing and purifying steps to obtain the resulting porous carbon.
Currently, it is widely used for the carbonization technique to prepare hierarchically nanoporous
carbons by selecting appropriate carbon sources or precursors. However, this kind preparation
method of nanoporous carbons is still unfavourable because there is always a very slow heating rate
with the conventional heating techniques (tube furnace), which makes the carbonization expensive
and difficult to scale up. Moreover, carbon materials prepared in this way can only generate similar
pore structures to the MOFs precursors, lacking variety if there is no other treatment. Microwave-
assisted heating, which arises from the direct interaction of matter with electromagnetic energy, has
attracted an increasing attention due to its potential advantages over traditional heating techniques
[24]. These advantages include a remarkable decrease in heat-up time, a smaller requirement of
energy, fewer steps involved in the heating process, a more sensitive and efficient operating system,
etc. The final samples obtained from the microwave-assisted heating method will most likely be
more economically competitive than those obtained using conventional methods [25].
In this work, we choose ZIF-8 as a precursor for the preparation of nanoporous carbons using a
microwave-assisted heating method. The approach presented here does not require any addition of
carbon sources or pore-forming templates. Furthermore, the surface area and pore structure are easily
regulated by controlling the microwave carbonization temperature. The resulting ZIF-derived
nanoporous carbons are comprehensively characterized using a varieties of techniques. It is discussed
in detail for the structural evolution of the nanoporous carbons with increasing temperature and their
electrochemical behaviour as electrode materials for supercapacitors.
2. Experimental section
2.1. Preparation of ZIF-8
ZIF-8 powder was synthesized on the basis of the method reported in the literature [26]. All
chemicals were purchased from Aladdin Chemical Co (Shanghai China). First, 2.62 g of zinc nitrate
hexahydrate (Zn (NO
3
)
2
·6H
2
O) was dissolved in 100 mL of methanol to form solution A, and 5.79 g
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
572

of 2-methylimidazole (MmIm) was dissolved in 100 mL of methanol to generate solution B. Then,
two solutions above were mixed up and stirred for 1 h at room temperature. After that, the resulting
milky solution was centrifuged at 10000 rpm for 5 min, and the supernatant was removed. The
precipitates was washed with methanol and centrifuged again. The whole process was repeated twice.
The resulting powder was dried in an oven at 60 ºC overnight.
2.2. Preparation of nanoporous carbon (NPC)
Five nanoporous carbons were prepared from direct carbonization of ZIF-8 using a microwave-
assisted heating method as follows: First, a quartz tube with 0.8 g of dried ZIF-8 powder was inserted
into an alumina crucible filled with SiC powder (a microwave absorber), which has a critical
auxiliary heating effect. Then, the whole alumina crucible was wrapped with asbestos and placed in a
microwave furnace. The furnace was heated up to the required carbonization temperature (600, 700,
800, 900 or 1000 °C) at a very high rate of heating (generally within 5 min) under a nitrogen
atmosphere with a gas pressure of 20 kPa. Then the target temperature was maintained for 3 h,
followed by cooling to room temperature in nitrogen. After treated with an aqueous solution of HCl
(0.1 M) to remove Zn or ZnO residue from the decomposition of ZIF-8 template, the as-prepared
nanoporous carbon materials were denoted as NPC-600, NPC-700, NPC-800, NPC-900, and NPC-
1000 respectively. For comparison, another carbon sample was obtained by direct carbonization of
ZIF-8 using a tube furnace. Firstly, the furnace was filled with nitrogen gas at room temperature.
Then, the furnace was heated up to 1000 °C at a conventional rate of 5 °C/min and held at 1000 °C
for 3 h. After the furnace was cooled to room temperature in nitrogen, the carbon sample was treated
with 0.1 M HCl and labelled as NPC-T-1000.
2.3. Characterization
X-ray diffraction (XRD) patterns were obtained on a Bruker D8 advance powder X-ray
diffractometer using Cu Kα radiation (20 kV, 200 mA, λ=1.54178 Å) with a step size of 0.02° and
step time of 0.5 s. Scanning electron microscopy (SEM) images were examined using a Hitachi SU-
70 scanning electron microscope at an acceleration voltage of 20 kV. Nitrogen adsorption-desorption
curves were recorded using a micrometrics ASAP 2020 instrument at liquid nitrogen temperature (77
K). All samples were degassed at 523 K for 4 h before measurement. The Brunauer-Emmett-Teller
(BET) surface areas were calculated from the nitrogen isotherm curves ranging from the relative
pressure of 0 to 0.3. The total pore volumes were determined from the amount of nitrogen adsorbed
at P/P
0
of ca. 0.99. The pore size distributions (PSDs) were determined using a density functional
theory (DFT) method. The micro- and mesopore volumes were obtained using adsorption isotherms
from a DFT method, and the macropore volumes were determined by subtracting the micro- and
mesopore volumes from the total pore volume. Transmission electron microscope (TEM) images
were obtained using a Joel JEM-2100 at an acceleration voltage of 100 kV. Laser Raman
spectroscopy was performed on a Renishaw inVia Reflex.
2.4. Electrode preparation and electrochemical performance test
All electrochemical performances were examined on a standard three-electrode electrochemical
station,
which was equipped with a counter electrode of Pt, a reference electrode of Hg/HgO, and a working
electrode of ZIF-derived nanoporous carbon in 6 M KOH. The working electrode was prepared by
mixing up ZIF-derived nanoporous carbon (85 wt%), acetylene black (10 wt%) and
polytetrafluoroethylene (5 wt%) with moderate ethanol to form slurry and then coat onto a nickel
foam with a coating area of 1 cm
2
, The foam was dried at 110 °C in vacuum oven overnight, Then, in
Hierarchical Porous Carbon Materials Derived from Microwave-assisted Heating of Zeolitic Imidazolate Frameworks for Use as
Supercapacitor Electrodes
573
order to completely adhere with the electrode material, the foam was pressed under a pressure of 10
MPa. The mass loading of active materials on the prepared working electrode is nearly 5 mg cm
-2
.
All electrochemical experiments were performed at ambient temperature on a CHI660e
electrochemical workstation (Shanghai Chenhua Instruments Co.). Before the measurements, the
working electrodes were soaked in electrolyte for half an hour so that the active materials could be
fully infiltrated by the electrolyte. The primary testing method was cyclic voltammetry (CV) at
different scan rates ranging from 5 to 200 mV s
-1
. Galvanostatic charge/discharge (GC)
measurements were also performed at different current densities raging from 0.1 A g
-1
to 10 A g
-1
in
the potential range of -1.0~0 V. Electrochemical impedance spectra (EIS) were measured over the
frequency ranging from 100 kHz to 0.01 Hz with an alternate current perturbation of 5 mV at open
circuit voltage. The EIS data were analysed using Nyquist plots with the real part (Z') and imaginary
part (Z'') of impedance marked as the X-axis and Y-axis, respectively.
For galvanostatic charge/discharge processes, the specific capacitances were calculated using the
following equation[27]: C
m
=IΔt(mΔV)
-1
. In this equation, C
m
(F g
-1
) is the specific capacitance, I (A)
represents the discharge current, Δt (s) represents the discharge time, m (g) refers to the mass of
active material on the electrode, and ΔV (V) is the voltage change within the discharge time.
3. Results and discussion
3.1. Structure and texture characterization
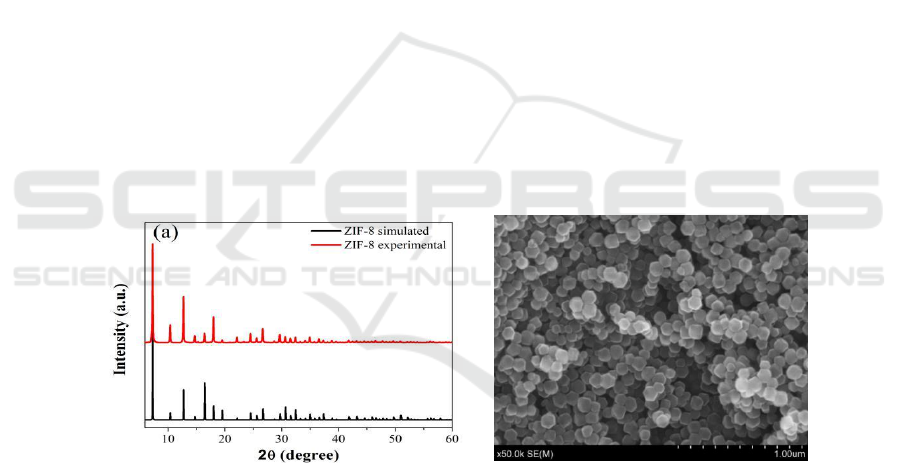
Figure 1 shows the XRD and SEM images of as-synthesized ZIF-8. The XRD pattern of ZIF-8
powder is in great agreement with the simulation, suggesting that the ZIF-8 material has been
synthesized successfully [28]. And the SEM image shows a typical rhombic dodecahedron
morphology of ZIF-8 with a particle size of approximately 100 nm.
Figure 1. (a) XRD pattern and SEM image of ZIF-8.
XRD patterns of NPC-600, NPC-700, NPC-800, NPC-900, NPC-1000 and NPC-T-1000 are
shown in Figure 2a. As we can see, after carbonization, all of the resulting porous carbon samples
lose the characteristic XRD peaks of the parental ZIF-8, and display two broad peaks at 2 = 23° and
44°, which are corresponding to the (002) and (101) planes of graphitic carbon. The (002) refers to a
graphite-like peak, and (101) is related to the honeycomb lattice in single-layer graphene [29, 30].
Two broad diffraction peaks of NPC-1000 are the most noticeable among six ZIF-derived
nanoporous carbon samples, indicating that NPC-1000 may hold better electrical conductivity and
crystalline degree.
(b)
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
574
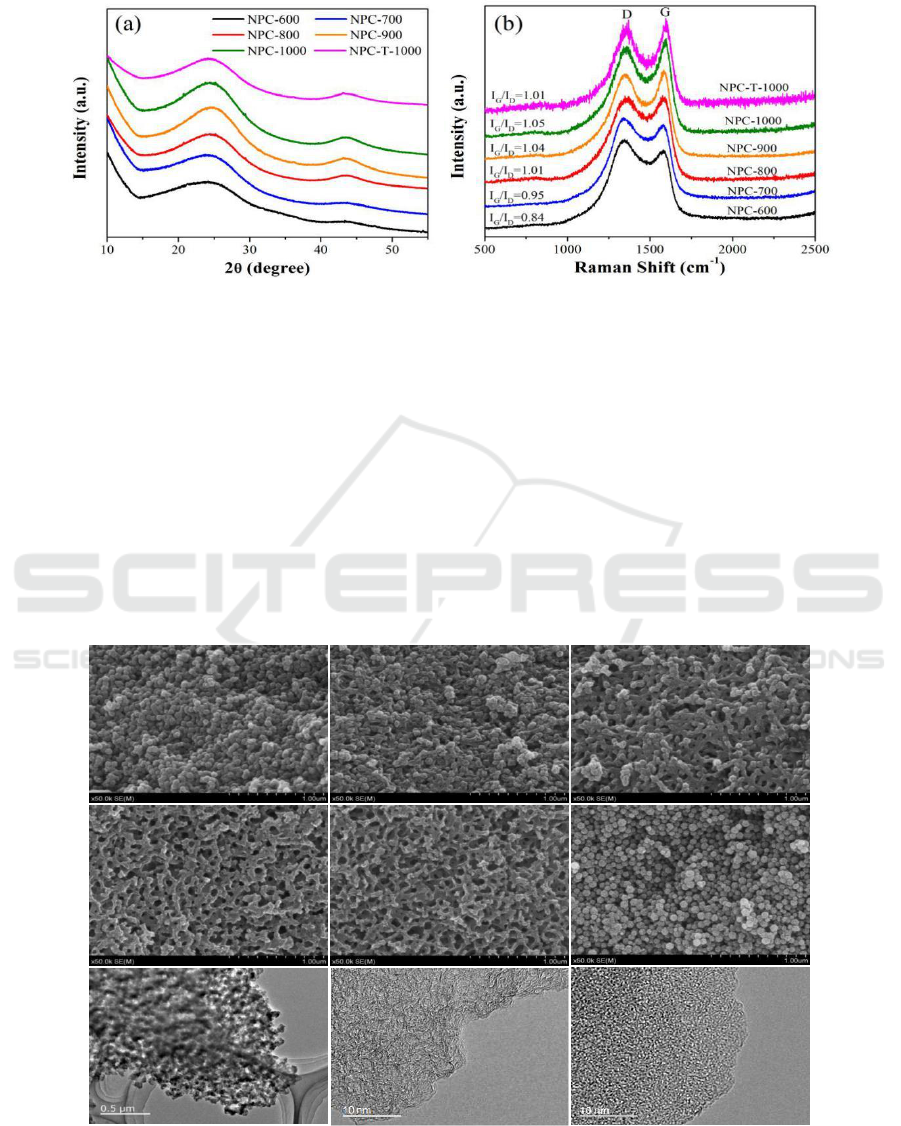
Figure 2. Powder XRD patterns and Raman spectra of six ZIF-derived nanoporous carbons.
To further study the graphitization degree of the six ZIF-derived nanoporous carbons, Raman
spectra are given in Figure 2b. There are two featured broad peaks centered at approximately 1580
cm
-1
and 1360 cm
-1
, which are named the G band (graphite) and D band (disorder) respectively. The
G band presents ideal graphitic sp
2
carbons, and the D band relates to disordered carbons [31, 32].
Generally, the graphitization degree of porous carbons can be estimated by comparing the relative
intensity ratio of the G band to the D band (I
G
/I
D
) [33]. The ZIF-derived nanoporous carbon samples
exhibited a gradual increase in I
G
/I
D
values from 0.84 to 1.05 as the microwave carbonization
temperature increased from 600 to 1000 °C, indicating that these nanoporous carbons possess both
graphitic and disordered structures [34] and a higher carbonization temperature contributes to the
generation of graphitic sp
2
carbons. In addition, NPC-T-1000 achieves a lower I
G
/I
D
value than NPC-
1000, which exhibits that microwaves are conducive to the formation of graphite carbon.
Figure 3. SEM images of six ZIF-derived nanoporous carbons: (a) NPC-600, (b) NPC-700, (c) NPC-
800, (d) NPC-900, (e) NPC-1000, (f) NPC-T-1000 and representative TEM images of NPC-1000 (g,
h) and NPC-T-1000 (i).
(h)
(a)
(c)
(d)
(e
)
(g)
(b)
(f)
(i)
Hierarchical Porous Carbon Materials Derived from Microwave-assisted Heating of Zeolitic Imidazolate Frameworks for Use as
Supercapacitor Electrodes
575

To explore the morphologies and structures of the six as-prepared ZIF-derived nanoporous carbon
materials, both SEM and TEM analyses were performed. As shown in Figure 3, apparent
morphological changes occur in the ZIF-derived nanoporous carbons. NPC-600 turns into spherical
particles with a decreased particle size of approximately 75 nm, which is similar to NPC-T-1000. As
the temperature increased to 800 °C , the sample cannot maintain the typical granular morphology
well and undergoes particle bonding, forming interconnections between particles. In particular, for
NPC-1000, instead of a typical granular morphology, a three-dimensional network morphology
derived from interconnected particles has been adopted, generating obvious mesopores in the range
of 30-40 nm and macropores in the range of 60-100 nm. Obviously, the morphologie and structure of
NPC-1000 is quite distinct from the carbon material prepared by direct carbonization of ZIF-8 using
a tube furnace [34, 35]. This indicates that the heating mode have important influence on the pore
structure of carbon materials and the microwave-assisted heating process can be explained as follows:
it generates a large amount of thermal energy when using microwave-assisted heating method to
carbonize ZIF-8 at a very fast heating rate. Thus, the organic species of ZIF-8 are quickly
decomposed, but the evacuating speed is far slower than the decomposing speed, so some of the
released linkers or atoms return to form bonds with each other, which appear as macroscopic particle
interconnections. As the temperature increased higher, large numbers of meso- and macropores
appear due to the large improvement in the evacuating and decomposing efficiency of ZIF-8.
Representative TEM images for NPC-1000 and NPC-T-1000 are presented in Figure 3 (g, h).
Mesopores and macropores are clearly visible in NPC-1000, and the high-resolution TEM reveals
that the sample possesses many micropores over the entire sample surface with a well-developed
graphitic structure while NPC-T-1000 is nearly amorphous. The relatively high graphitization degree
of NPC-1000 makes it a better electrical conductivity, which play an important role in
electrochemical performance.
The nitrogen adsorption-desorption isotherms at 77 K (Figure 4a) were used to measure the
specific surface areas and nanopores of the obtained porous carbons. At low relative pressure
(P/P
0
<0.1), all six porous carbons display significant nitrogen adsorption, which is in agreement with
type I adsorption-desorption isotherm, indicating the development of microporosity. The isotherms
for all the porous carbons are extremely similar, and the adsorption-desorption isotherm branches are
almost reversible, indicating that all the carbons are predominantly microporous, which is
corresponding to micropore structures presented in the parental ZIF-8 that acts as both the template
and the carbon precursor [35]. In particular, for NPC-900 and NPC-1000, there appear hysteresis
loops at high relative pressure (P/P
0
>0.8), indicating the existence of partial mesopores or
macropores in the carbons, which is in great agreement with the SEM and TEM results. It should be
noted that NPC-T-1000 give the isotherm with a larger hysteresis loop than that of NPC-1000 over
the relative pressure from 0.8 to 1.0. This is come from the interparticle space among the assembled
small-sized particles rather than porosity in the material itself, which is invalid in electrochemical
behaviour. The textural properties of the carbons are listed in Table 1. The BET surface areas and
total pore volumes of six carbon samples are in the ranges of 384.4-1281.4 m
2
g
-1
and 0.17-0.87 cm
3
g
-1
. Among them, NPC-T-1000 shows the highest surface area of 1281.4 m
2
g
-1
, and the largest total
pore volume of 0.87 cm
3
g
-1
due to the rich intergranular space. In comparison, NPC-1000 possesses
lower values of 852 m
2
g
-1
and 0.49 cm
3
g
-1
respectively. The proportion of micropore surface area
for the carbon materials using micro-assisted heating method is approximately 95%, and the
proportion of mesopore and macropore volume varies from 35% to 41%. The relatively low
micropore contributions for NPC-900 and NPC-1000 can be attributed to the complete
decomposition of ZIF-8 and the formation of particle interconnections at high microwave
carbonization temperature, leading to the formation of mesopores and macropores. NPC-1000 has the
largest proportion of mesopore and macropore volume of 41%, which may make the sample a faster
electron transfer.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
576
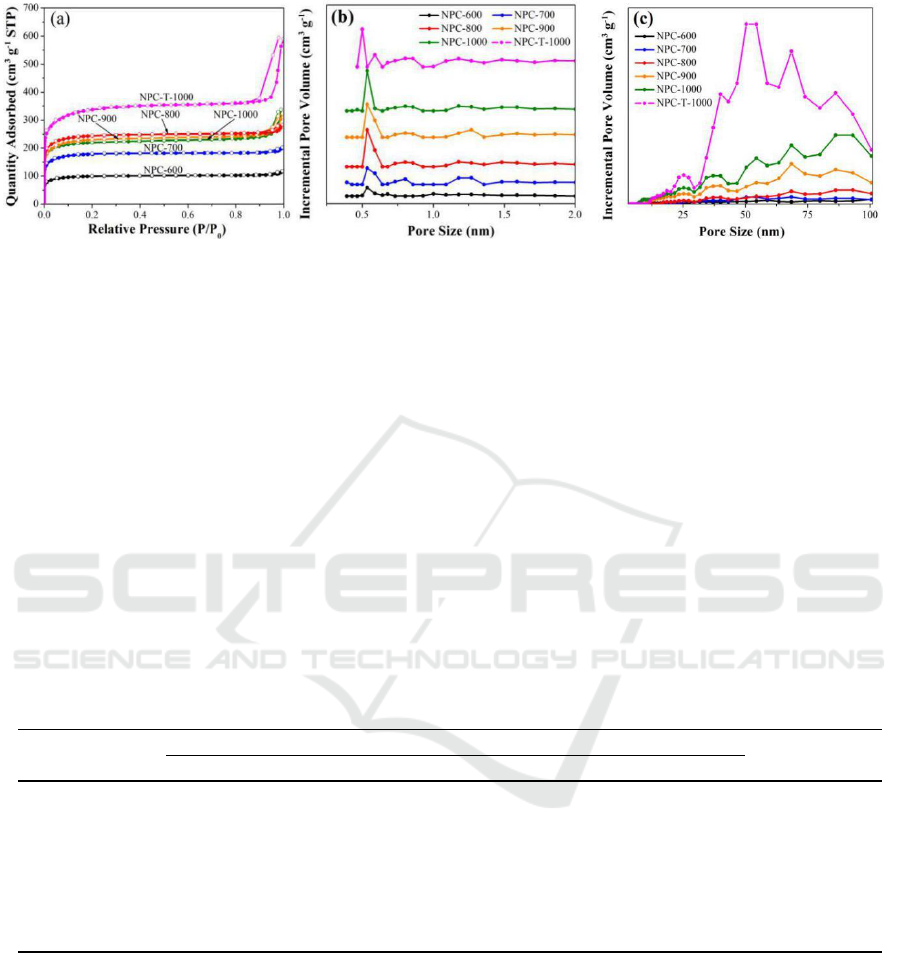
Figure 4. N
2
adsorption-desorption isotherms (a) and the corresponding pore size distributions of
the ZIF-derived nanoporous carbons at different temperature, (b) micropore size distribution, (c)
meso- and macropore size distribution.
To obtain more detailed information about the nanoporous structures, the pore size distributions
(PSDs) (Figure 4b, c) were calculated using density functional theory based on the N
2
adsorption data.
Five ZIF-derived nanoporous carbons using microwave-assisted heating exhibit narrow and sharp
micropore size distribution centred at approximately 0.6 nm, while NPC-T-1000 suffers a smaller
micropore size less than 0.5 nm. Small micropores may lead to low efficiency in capacitive
performance, NPC-900 and NPC-1000 also possess macropores larger than 50 nm and mesopores of
20-50 nm. There are some other differences among the materials carbonized at different microwave
carbonization temperatures. The PSD peaks of the samples carbonized at high temperature are higher
than those of the low-temperature carbonized samples, and the micropore size of the carbons is
slightly increased with increasing microwave carbonization temperature, indicating that the
microwave holding temperature has a significant effect not only on the surface area and pore volume
but also on the pore diameter and amount.
Table 1. Surface area, pore volume and capacitance of ZIF-derived nanoporous carbons.
Samples
surface area (m
2
g
-1
)
Pore volume (cm
3
g
-1
)
C
m
f
(F g
-1
)
S
BET
a
S
micro
b
V
t
c
V
micro
d
V
meso/macro
e
NPC-600
384.4
367
0.17
0.11
0.06
109.6
NPC-700
693.1
669.4
0.30
0.23
0.07
149.9
NPC-800
947.5
906
0.42
0.32
0.1
194.3
NPC-900
886.9
832.2
0.47
0.29
0.18
201.1
NPC-1000
852
799.1
0.49
0.29
0.2
207.7
NPC-T-1000
1281.4
1161.9
0.87
0.41
0.46
181.5
a
Brunauer-Emmett-Teller (BET) surface area.
b
Microporous surface area.
c
Total pore volume at P/P
0
=0.99.
d
Micropore volume.
e
Mesopore and macropore volume.
f
Specific capacitance at a current density of 0.1 A g
-1
.
3.2. Electrochemical analysis
To investigate the capacitive performance of the six ZIF-derived nanoporous carbons, the CV, GC,
and EIS were measured. The CV curves of the ZIF-derived nanoporous carbons at a scan rate of 25
mV s
-1
are illustrated in Figure 5a, the cyclic voltammetry curves for all ZIF-derived nanoporous
Hierarchical Porous Carbon Materials Derived from Microwave-assisted Heating of Zeolitic Imidazolate Frameworks for Use as
Supercapacitor Electrodes
577

carbons exhibit a nearly rectangular shape, which is typical behaviour for supercapacitors [36,37].
Compared to all other carbon samples, NPC-1000 shows the largest CV area and a more rectangular
shape. The GC curves (Figrue 5b) at a current density of 0.1 A g
-1
are symmetric, and NPC-1000
possesses the longest discharge time. The specific capacitances of the six nanoporous carbons are
listed in Table 1, these values were calculated according to the discharge process. Obviously, NPC-
1000 presents the largest capacitance value of 207.7 F g
-1
compared to other porous carbons. This
result presumably arises from both the higher percentage of graphitic carbons and the suitable
hierarchical micro/meso/macropores structure of NPC-1000, which are advantageous for electrical
conductivity and quick diffusion of electrolyte to the electrode [38-40]. It must be noted that although
NPC-T-1000 possesses the largest value of surface area, its capacitance is only 181.5 F g
-1
, which is
even lower than the values of NPC-800 and NPC-900. The phenomenon reveals that capacitance is
not in direct proportion to the specific surface area, there is only part of micropores are in effective
use when the charge is transfered.
Figure 5c shows the electrochemical impedance spectroscopy, providing available details about
the frequency responses of six ZIF-derived nanoporous carbons as supercapacitor electrode materials.
In the high frequency region, the Nyquist plots exhibit depressed semicircles for the carbons (see the
inset of Figrue 5c), which is related to the charge transport resistance between the electrode and
electrolyte [41]. NPC-1000 shows a smallest crossing value of the Z’ axis, which indicates that NPC-
1000 has lower interfacial contact resistance, because NPC-1000 possesses a hierarchical pore
structure, which is favourable for electrolyte ion migration. In the low frequency region, the larger
the curve slope, the better the capacitive behaviour. A vertical curve indicates ideal capacitive
behaviour and a fast transportation of the electrolyte ions in the nanopores of the carbon materials
[42]. A shift of the vertical curve could result from the Warburg resistance for the electrolyte ions.
Obviously, the EIS curve for NPC-1000 shows the largest value of curve slope, i.e., the lowest
Warburg resistance, which demonstrates that NPC-1000 possesses the best electrical properties
among the nanoporous carbons to act as an electrode material. The specific capacitances of six ZIF-
derived nanoporous carbons at different current densities are presented in Figure 5d. Obviously,
NPC-1000 shows the best capacitive performance. Detailedly, NPC-1000 shows the highest
capacitance of 207.7 F g
-1
at 0.1 A g
-1
. Moreover, the capacitance of NPC-1000 still keep 66.5%
when increasing the current density from 0.1 to 10A g
-1
. In comparison, NPC-T-1000 achieves lower
values of 181.5 F g
-1
and 60.1% under the same test conditions. Obviously, NPC-1000 achieves the
largest retention among the six nanoporous carbon samples.
To further study the electrochemical performance of NPC-1000, we performed a series of
capacitive measurements at different current densities and different scan rates (Figure 6). Figure 6a
exhibits the CV curves of NPC-1000 at different scan rates of 5, 10, 25, 50, 100 and 200 mV s
-1
, all
of them are in typical rectangular shape. At 200 mV s
-1
, the CV curve suffers from some slight
distortion, which may result from the limited mass transfer or ion transport [43]. As shown in Figure
6b, the triangle charge/discharge curves of NPC-1000 exhibit almost linear and symmetrical shapes
at different current densities ranging from 0.1 to 10 A g
-1
, indicating that the NPC-1000 electrode
possesses good electrochemical reversibility [44]. The capacitance of NPC-1000 reaches 207.7 F g
-1
at 0.1 A g
-1
. It must be noted that this value is much larger than the ones of ZIF-8-derived nanoporous
carbons using traditional carbonization method, like Z-800 derived from direct carbonization of ZIF-
8 carbonized at 800 °C (130F g
-1
at a scan rate of 50 mV s
-1
) and Z-1000 at 1000 °C (112F g
-1
at a
scan rate of 50 mV s
-1
) [31]. In addition, it was investigated and shown in Figure 6c for long-term
cycle stability of NPC-1000 at a current density of 5 A g
-1
. The capacitance of NPC-1000 keeps
almost unchanged and only 4.7% of capacitance is lost after 1000 cycles, indicating that the material
structures of NPC-1000 are very stable in the charge and discharge process.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
578
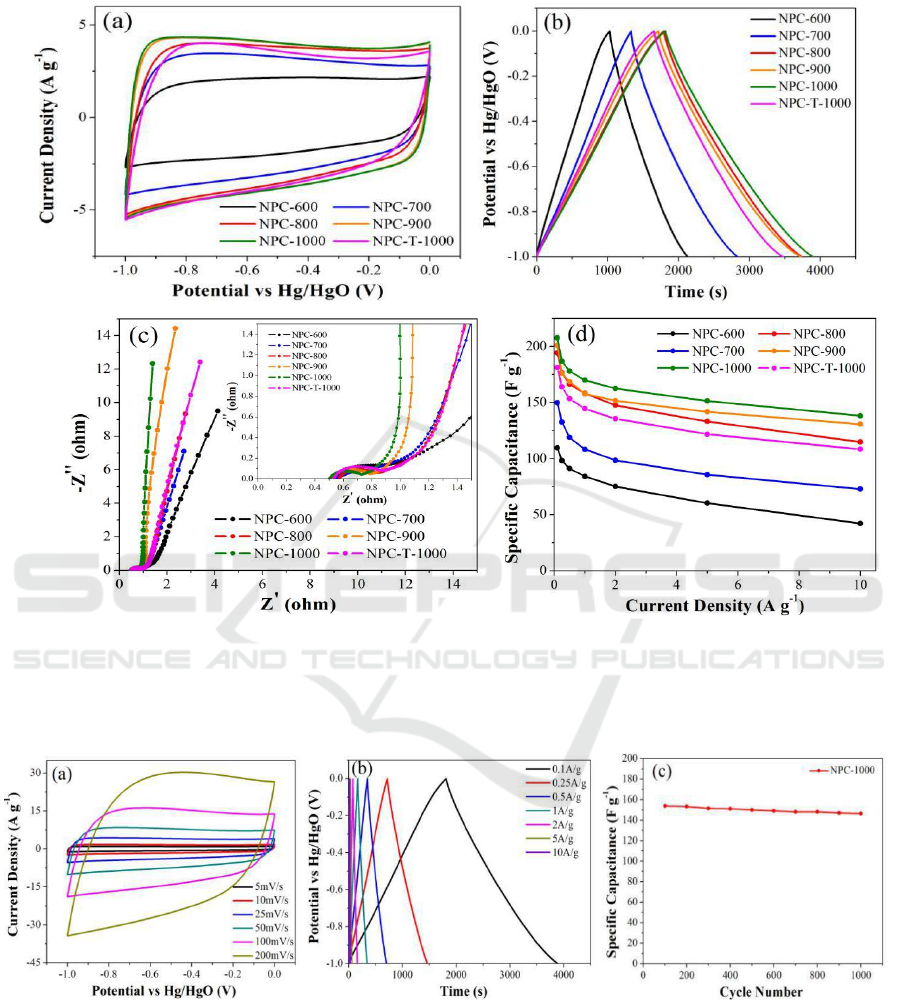
Figure 5. Electrochemical properties of ZIF-derived nanoporous carbons. (a) Cyclic voltammetry
curves at 25 mV s
-1
, (b) galvanostatic charge and discharge curves at 0.1 A g
-1
, (c) impedance
spectroscopy, and (d) specific capacitance at different current densities.
Figure 6. Capacitive performance of NPC-1000. (a) Cyclic voltammetry curves at different scan
rates, (b) galvanostatic charge and discharge curves at different current densities, and (c) cycling
stability at 5A g
-1
.
Hierarchical Porous Carbon Materials Derived from Microwave-assisted Heating of Zeolitic Imidazolate Frameworks for Use as
Supercapacitor Electrodes
579

Table 2. MOFs derived nanoporous carbons for supercapacitor.
Samples
BET
(m
2
g
-1
)
Electrolyte
Scan rate
(mV s
-1
)
Current
density
(A g
-1
)
Capacitance
(F g
-1
)
Ref.
NPC-1000
838
6 M KOH
0.1
207.7
this work
Z-700
520
0.5M H
2
SO
4
50
—
23
31
Z-800
720
0.5M H
2
SO
4
50
—
130
31
Z-900
1075
0.5M H
2
SO
4
50
—
128
31
Z-1000
1110
0.5M H
2
SO
4
50
—
112
31
MC
1812
6M KOH/1.5M,
NEt
4
BF
4
acetonitrile
—
0.25
149/113
44
C800
2169
1 M H
2
SO
4
5
—
188
45
C1000
3405
1 M H
2
SO
4
5
—
161
45
C-MOF-5
2119
1 M H
2
SO
4
—
1
150
46
C-MOF-2
1378
1 M H
2
SO
4
—
1
170
46
C-Zn-BTC
1326
1 M H
2
SO
4
—
1
134
46
C-Zn-NDC
920
1 M H
2
SO
4
—
1
114
46
C-Zn-ADA
513
1 M H
2
SO
4
—
1
95
46
C-Zn-PAA
495
1 M H
2
SO
4
—
1
110
46
4. Conclusions
In summary, we have used, for the first time, a microwave-assisted heating technique to efficiently
prepare hierarchical porous carbon materials via the direct carbonization of ZIF-8 without any
additional carbon sources. In this study, the surface areas and pore structures of the resulting carbons
were easily controlled by the microwave carbonization temperature. The obtained ZIF-derived
nanoporous carbons exhibit specific surface areas in the range of 384.4-947.5 m
2
g
-1
and pore
volumes in the range of 0.17-0.49 cm
3
g
-1
. These porous carbon materials are mainly composed of
micropores, but certain amounts of mesopores and macropores emerge due to the interconnection of
nanoparticles when the carbonization temperature is higher than 800 °C, which is quite different from
carbon material prepared using traditional heating techniques. Of all the ZIF-derived nanoporous
carbons examined, NPC-1000 possesses the highest percentage of mesopores and macropores and
achieves the highest capacitance of 207.7 F g
-1
at a current density of 0.1 A g
-1
, due to its higher
percentage of graphitic carbons and proper hierarchical pore structure. Moreover, NPC-1000 also
shows excellent rate capability as a capacitor electrode material. The capacitance retention of NPC-
1000 is 66.5% when the current density changes from 0.1A g
-1
to 10A g
-1
. Therefore, microwave-
assisted heating is a very promising technique for the synthesis of MOF-derived nanoporous carbons.
In particular, it can be used as a reference route for the preparation of hierarchical porous carbon
materials. Future work will focus on improvements to provide improved microwave power control
and allow the carbonization of other MOF precursors.
Acknowledgment
We thank the editor and reviewers for their valuable comments and suggestions and Shenzhen
University for the financial support. This research work was supported by National Natural Science
Foundation of China (Nos. 51202150 and 51272161), Science and Technology R&D Program of
ShenZhen (JCYJ20150324141711663), Program of Introducing Innovative Research Team in
Dongguan (No.2014607109) and Open Foundation of Yanshan University Aviation Key Laboratory
of Science and Technology on Generic Technology of Self-Lubricating Spherical Plain Bearing
(No.HKKJ20140802).
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
580

References
[1] Winter M and Brodd R J 2004 What are batteries, fuel cells, and supercapacitors? Chem Rev.
104(10) 4245-70
[2] Zhao Y, Liu J, Hu Y, Cheng H, Hu C, Jiang C and et al 2013 Highly compression-tolerant
supercapacitor based on polypyrrole-mediated graphene foam electrodes Adv Mater. 25(4)
591-5
[3] Wang D W, Liu S J, Jiao L, Fang G L, Geng G H and Ma J F 2017 Unconventional mesopore
carbon nanomesh prepared through explosion-assisted activation approach: a robust
electrode material for ultrafast organic electrolyte supercapacitors Carbon. 119 30-9
[4] Wang D W, Liu S J, Fang G L, Geng G H and Ma J F 2016 From trash to treasure: direct
transformation of onion husks into three-dimensional interconnected porous carbon
frameworks for high-performance supercapacitors in organic electrolyte Electrochim Acta.,
216 405-11
[5] Borchardt L, Oschatz M and Kaskel S 2014 Tailoring porosity in carbon materials for
supercapacitor applications Mater Horiz. 1(2) 157-68
[6] Wang D W, Liu S J, Jiao L and Fang G L 2017 A smart bottom-up strategy for the fabrication
of porous carbon nanosheets containing rGO for high-rate supercapacitors in organic
electrolyte Electrochim Acta. 252 109-18
[7] Wang H and Gao Q 2009 Synthesis, characterization and energy-related applications of
carbide-derived carbons obtained by the chlorination of boron carbide Carbon. 47(3) 820-8
[8] Wang D W, Li F, Liu M, Lu G Q and Cheng H M 2008 3D aperiodic hierarchical porous
graphitic carbon material for high-rate electrochemical capacitive energy storage Angew
Chem. 47 373-6
[9] Wang D W, Fang G L, Geng G H and Ma J F 2017 Unique porous carbon constructed by
highly interconnected naonowalls for high-performance supercapacitor in organic
electrolyte Mater Lett., 189 50-3
[10] Xia K, Gao Q, Jiang J and Hu J 2008 Hierarchical porous carbons with controlled micropores
and mesopores for supercapacitor electrode materials Carbon. 46, 1718-26.
[11] Largeot C, Portet C, Chmiola J, Taberna P L, Gogosti Y and Simon P 2008 Relation between
the ion size and pore size for an electric double-layer capacitor J Am Chem Soc. 130 2730-1.
[12] Wang D W, Wang Y T, Liu H W, Xu W and Xu L 2018 Unusual carbon nanomesh
constructed by interconnected carbon nanocages for ionic liquid-based supercapacitor with
superior rate capability Chem Eng J. 342 474-83
[13] Thess A, Lee R, Nikolaev P, Dai H, Petit P, Robert J and et al 1996 Crystalline ropes of
metallic carbon nanotubes Science. 273(5274) 483-7
[14] Journet C, Maser W K, Bernier P, Loiseau A, Chapelle M L, Lefrant S and et al 1997 Large-
scale production of single-walled carbon nanotubes by the electric-arc technique Nature.
388(6644) 756-8
[15] Zheng B, Lu C, Gu G, Makarovski A, Finkelstein G and Liu J 2002 Efficient CVD growth of
single-walled carbon nanotubes on surfaces using carbon monoxide precursor Nano Lett.
2(8) 895-8
[16] Yang Q, Xu W, Tomita A and Kyotani T 2005 The template synthesis of double coaxial
carbon nanotubes with nitrogen-doped and boron-doped multiwalls J Am Chem Soc. 127(25)
8956-7
[17] Kim T W, Park I S and Ryoo R 2003 Intramolecular aromatic amination through iron-
mediated nitrene transfer. Angew Chem Int Ed. 42(36) 4360-75
[18] Ahmadpour A and Do D D 1996 The preparation of active carbons from coal by chemical and
physical activation Carbon. 34(4) 471-9
Hierarchical Porous Carbon Materials Derived from Microwave-assisted Heating of Zeolitic Imidazolate Frameworks for Use as
Supercapacitor Electrodes
581

[19] Fuertes A B 2003 Template synthesis of mesoporous carbons with a controlled particle
size J Mater Chem. 13 3085-8
[20] Ma C, Shao X and Cao D 2012 Nitrogen-doped graphene nanosheets as anode materials for
lithium ion batteries J Mater Chem. 22 8911-5
[21] Zhang P, Sun F, Xiang Z, Shen Z, Yun J and Cao D 2014 ZIF-derived in situ nitrogen-doped
porous carbons as efficient metal-free electrocatalysts for oxygen reduction reaction Energy
Environ Sci. 7(1) 442-50
[22] Liu B, Shioyama H, Akita T and Xu Q 2008 Metal-organic framework as a template for
porous carbon synthesis J Am Chem Soc. 130(16) 5390-1
[23] Schoenecker P M, Carson C G, Jasuja H, Flemming C J and Walton K S 2012 Effect of water
adsorption on retention of structure and surface area of metal-organic frameworks Ind Eng
Chem Res. 51(18) 6513-9
[24] National Research Council National Materials Advisory Committee Microwave processing of
materials National Research Council Washington DC National Academy Press 1994
[25] Menéndez J A, Arenillas A, Fidalgo B, Fernández Y, Zubizarreta L, Calvo E G and et al 2010
microwave-assisted heating processes involving carbon materials Fuel Process. Technol. 91
1-8
[26] Maruyama J, Sumino K, Kawaguchi M and Abe I 2004 Influence of activated carbon pore
structure on oxygen reduction at catalyst layers supported on rotating disk electrodes
Carbon. 42(15), 3115-21
[27] Sun L, Tian C, Fu Y, Yang Y, Yin J, Wang L and et al 2014 Nitrogen-Doped Porous Graphitic
Carbon as an Excellent Electrode Material for Advanced Supercapacitors Chem Eur J. 20(2)
564-74
[28] Aleksandra S, Lavinia B, Véronique F, Lionel A, Ghouti M and Raphaël S 2014 Controlling
ZIF-8 nano- and microcrystal formation and reactivity through zinc salt variations
CrystEngComm. 16 4493-500
[29] Fletcher E A 1999 Solarthermal and solar quasi-electrolytic processing and separations: zinc
from zinc oxide as an example Ind Eng Chem Res. 38(6) 2275-82
[30] Yang S J, Kim T, Im J H, Kim Y S, Lee K, Jung H and et al 2012 MOF-derived hierarchically
porous carbon with exceptional porosity and hydrogen storage capacity Chem Mater. 24(3)
464-70
[31] Zhang J T, Yu L and Lou X W 2017 Embedding CoS2 nanoparticles in N-doped carbon
nanotube hollow frameworks for enhanced lithium storage properties Nano Res 10(12)
4298-304
[32] Chen L F, Lu Y, Yu L and Lou X W 2017 Designed formation of hollow particle-based
nitrogen-doped carbon nanofibers for high-performance supercapacitors Energ Environ Sci.
10 1777
[33] Rakhi R B, Chen W, Cha D and Alshareef H 2012 Nanostructured ternary electrodes for
energy-storage applications Adv Energy Mater. 2(3) 381-9
[34] Chaikittisilp W, Hu M, Wang H J, Huang H S, Fujita T, Wu K C W and etal 2012 Nanoporous
carbons through direct carbonization of a zeolitic imida zolate framework for
supercapacitor electrodes Chem. Commun. 48 7259-61
[35] Bai F H, Xia Y D, Chen B L, Su H Q and Zhu Y Q 2014 Preparation and carbon dioxide
uptake capacity of N-doped porous carbon materials derived from direct carbonization of
zeolitic imidazolate framework Carbon. 75 213-26
[36] Wang Q, Yan J, Wang Y, Wei T, Zhang M, Jing X and et al 2014 Threedimensional flower-
like and hierarchical porous carbon materials as high-rate performance electrodes for
supercapacitors Carbon. 67 119-27
[37] Torad N L, Salunkhe R R, Li Y, Hamoudi H, Imura M, Sakka Y and et al 2014 Electric
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
582

double-layer capacitors based on highly graphitized nanoporous carbons derived from ZIF-
67 Chem Eur J. 20 7895-900
[38] Sun L, Tian C, Fu Y, Yang Y, Yin J, Wang L and et al 2014 Nitrogendoped porous graphitic
carbon as an excellent electrode material for advanced supercapacitors Chem Eur J. 20(2)
564-74
[39] Yang S J, Kim T, Lee K, Kim Y S, Yoon J and Park C R 2014 Solvent evaporation mediated
preparation of hierarchically porous metal organic framework-derived carbon with
controllable and accessible large-scale porosity Carbon. 71 294-302
[40] Wu H, Wang X, Jiang L, Wu C, Zhao Q, Liu X and et al 2013 The effects of electrolyte on the
supercapacitive performance of activated calcium carbide-derived carbon J Power Sources
226 202-9
[41] Wu X, Hong X, Luo Z, Hui K S, Chen H, Wu J and et al 2013 The effects of surface
modification on the supercapacitive behaviors of novel mesoporous carbon derived from
rod-like hydroxyapatite template Electrochim Acta. 89 400-6.
[42] Ma C, Shao X and Cao D 2012 Nitrogen-doped graphene nanosheets as anode materials for
lithium ion batteries. J Mater Chem. 22 8911-5.
[43] Li Y, Roy S, Ben T, Xu S and Qiu S 2014 Micropore engineering of carbonized porous
aromatic framework (PAF-1) for supercapacitors application Phys Chem Chem Phys. 16(25)
12909-17
Hierarchical Porous Carbon Materials Derived from Microwave-assisted Heating of Zeolitic Imidazolate Frameworks for Use as
Supercapacitor Electrodes
583
