
Morphology and Diffusion Behaviour of Nafion and SPEEK
R J Wang, M Li
*
, C J Liu, X Q He and X D You
Department of Environmental Science & Engineering, North China Electric Power
University, Baoding, China
Corresponding author and e-mail: M Li, ming2999@126.com
Abstract. Phase separation morphology and diffusion properties of Nafion and SPEEK were
investigated in this study. Nafion and SPEEK PEMs had been prepared by solution casting
procedure. TEM image of Nafion membrane shows a preferable continuous proton
conductive channels with 3-5 nm width distributed in hydrophobic matrix, while SPEEK
exhibits poor phase separation morphology with isolated clusters (1 nm in width)
morphology. Radial distribution function of S-S for Nafion exhibits a much more intensive S-
S peak than that of SPEEK, suggesting that the agglomeration of hydrophilic domains,
represented by sulfonate groups, in SPEEK is worse than that in Nafion matrix, which might
be attributed to the lower electronegativity of H than that of F and higher steric hindrance of
SPEEK backbone. The corresponding hydrophilic cluster morphology of Nafion and SPEEK
membranes with different hydration level were explored by MD simulation. SPEEK
possessed much smaller hydrophilic channels than that of Nafion especially at low hydration
level. And consequently, much lower mobility of H
2
O and H
3
O
+
are revealed for SPEEK.
1. Introduction
Proton exchange membrane fuel cell (PEMFC) has drawn increasing attention for their high
efficiency and zero emission [1, 2]. Acting as a key component of PEMFC, proton exchange
membrane (PEM) has been intensively investigated [3].
Perfluorosulfonated ionomers (PFSI), in particular Nafion with equivalent weight (EW) of 1100,
are the most widely used proton-exchange membrane (PEM) materials because of good stability and
proton conductivity. However, low operation temperature, high cost and high fuels crossover limited
its further application. As one of the promising alternatives for Nafion, sulfonated
polyetheretherketone (SPEEK) possesses good chemical stability and thermal stability, low cost, high
mechanical stability, but poor hydrophilic cluster morphology and insufficient proton conductivity
[4-6].
It’s well known that the inherent proton diffusion of PEM is dominated by the chemical
configuration, hydrophilic/hydrophobic phase separation and water content [7-9]. It's crucial to
explore the difference in the morphologies and properties between Nafion and SPEEK materials.
Molecular dynamic (MD) simulation is a powerful tool to investigate the structure and diffusion
information in molecular scale that could not be easily obtained using experimental methods.
Recently, MD simulation has been successfully applied to explore the membrane structure, water
cluster distribution and transport of proton and water in PEMs [10-13]. In spite of the significant
experimental and theoretical attempts performed, the comparison on the structure and diffusion
552
Wang, R., Li, M., Liu, C., He, X. and You, X.
Morphology and Diffusion Behaviour of Nafion and SPEEK.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 552-557
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

properties of perfluoro-based Nafion and hydrocarbon-based SPEEK have not been implemented in
previous efforts, and the detailed mechanism of proton transport in PEMs is still unclear. Therefore,
the morphology and diffusion behavior of Nafion and SPEEK were investigated experimentally and
theoretically.
Nafion (EW=1100) was adopted in this study. SPEEK with higher degree of sulfonation (DS)
shows better hydrophilic cluster morphology and higher proton conductivity. However, excessive
hydrophilic characteristic results in swelling and poor mechanical properties. Our preliminary study
confirmed that EW 632 SPEEK obtained good stability and performance [14]. This study aims to
investigate the phase separation morphology and diffusion behavior of Nafion and SPEEK under
different water state in terms of experiment and molecular dynamic (MD) simulation.
2. Experiment and molecular simulation
2.1. Preparation of Nafion and SPEEK membranes
Nafion solution (20 wt. %, EW=1100) was provided by Aldrich. Poly (etheretherketone) (PEEK,
VESTAKEEP4000P with density of 1.30 g cm
3
) powder was supplied by Degussa. SPEEK
(EW=632, DS=50%) was prepared from poly (etheretherketone) (PEEK) according to our previous
work [5]. Solution casting method was applied to fabricate Nafion and SPEEK membranes [7, 14].
2.2. Characterization methods
Nafion and SPEEK membranes were characterized on a FEI Tecnai G2-TF30 transmission electron
microscope (TEM).
2.3. Molecular Simulation
MD simulations of Nafion and SPEEK systems were conducted to investigate the difference in the
hydrophilic cluster morphology and the diffusion properties between Nafion and SPEEK.
Nafion (EW=1100) and SPEEK (EW=632 EW, DS=50%) were built in this study. Nafion chain
of 10 units were adopted. Each SPEEK chain consisted of 5 sulfonated PEEK monomers and 5
PEEK monomers. Periodic cell of Nafion and SPEEK at different hydration level (from 0 to 14,
where λ= (H
2
O, H
3
O
+
)/SO
3
-
) were constructed. Both sulfonate groups of Nafion and SPEEK chains
are undissociated when hydration level is 0. In the contrary, sulfonate groups of other systems with
hydration level above 0 are dissociated. Table 1 exhibits the composition of each periodic cell of
SPEEK with different hydration levels. According to literature, the initial density is set to be 2 g cm
−3
for Nafion system. Due to chain rigidity and stiffness of SPEEK, an initial density of 0.4 g cm
−3
is set
for the periodic cell to avoid ring spearing and catenation.
Table 1. Construction of periodic cells of SPEEK with different hydration levels.
Hydration level Number of SPEEK chain Number of H
3
O
+
Number of H
2
O
0 5 0 0
1 5 25 0
2 5 25 25
4 5 25 75
6 5 25 125
8 5 25 175
10 5 25 225
14 5 25 325
Morphology and Diffusion Behaviour of Nafion and SPEEK
553
After system construction, to fully equilibrate the amorphous cells, anannealing procedure were
carried out on the constructed atomistic structures. Annealing strategy adopted in this study was
shown in Figure 1. Finally, 1 ns NVT ensemble was further conducted at 300K, the trajectory
information were recorded for structural and dynamic analysis.
Figure 1. Simulation strategy used in this study.
3. Results and discussion
3.1. Cluster Morphology of Nafion and SPEEK membranes
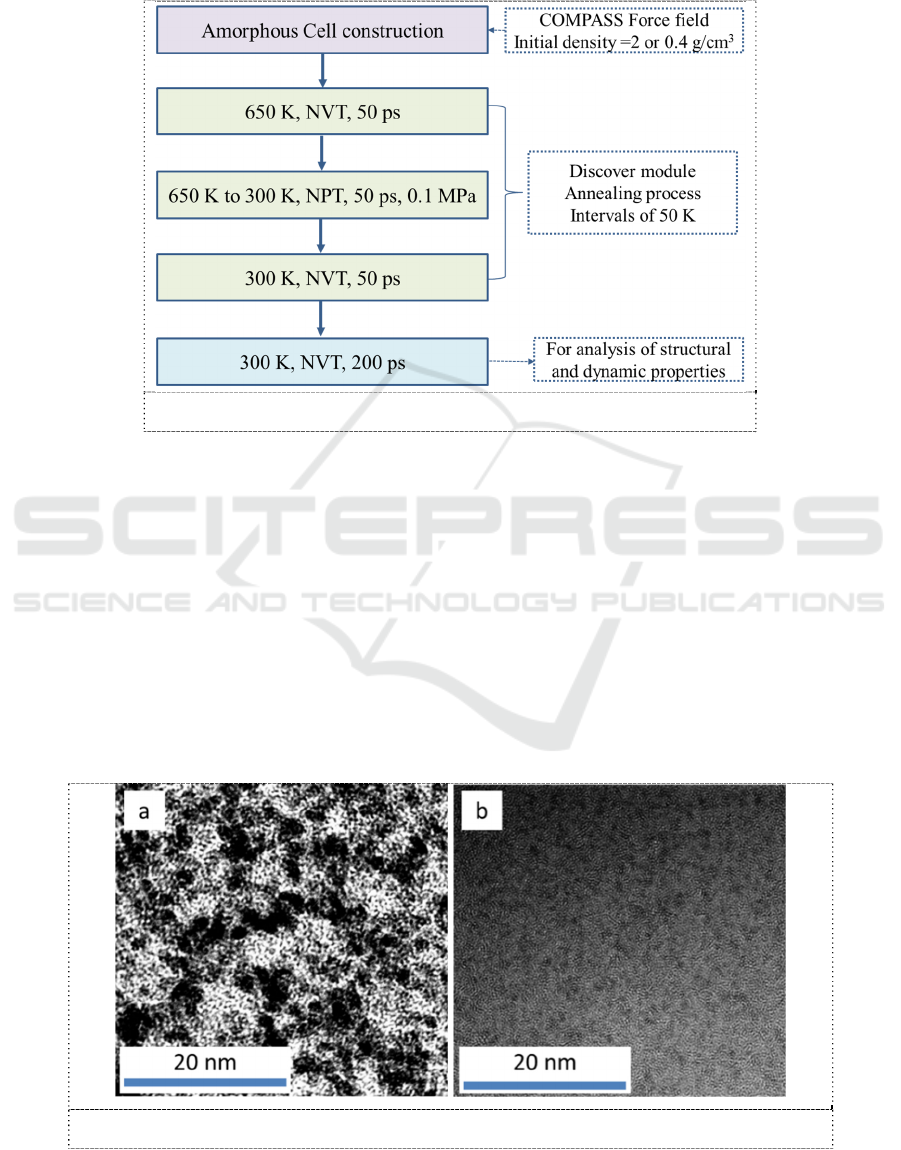
Cluster morphology of PEM dominated its electrochemical behaviors. Therefore, the morphologies
of Nafion and SPEEK were investigated in terms of TEM. As shown in Figure 2a, good phase
separation morphology is captured in Nafion membrane. The clusters with 3-5 nm width (black
spots) are distributed in hydrophobic matrix (white region), showing a preferable continuous proton
conductive channels. Compared with Nafion membrane, SPEEK exhibits poor phase separation
morphology in Figure 2b. The contrast between hydrophilic clusters and hydrophobic matrix is
relatively low. Isolated clusters with about 1 nm width were detected for SPEEK membrane. Since
the proton conduction is greatly influenced by the hydrophilic cluster morphology, a lower proton
conductivity than that of Nafion is suggested for SPEEK PEM.
Figure 2. TEM images of Nafion and SPEEK PEMs. (a) Nafion and (b) SPEEK.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
554
3.2. Validation of molecular dynamic simulations for Nafion and SPEEK systems
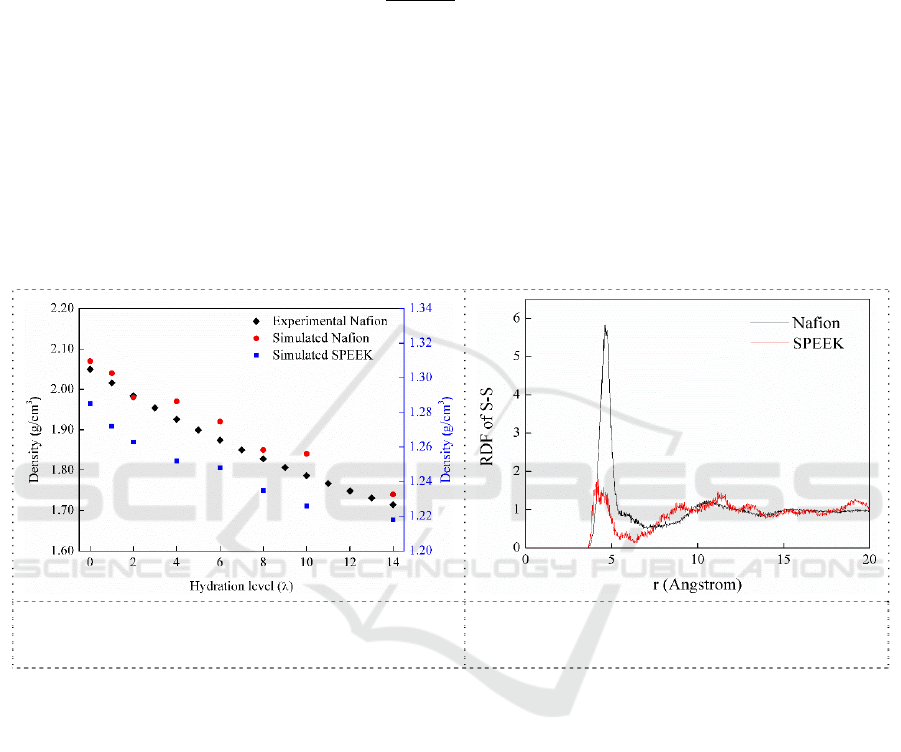
To validate the simulation result, experimental densities and simulated densities of Nafion at
different hydration level are plotted in Figure 3. The experimental densities of Nafion plotted in the
figure are calculated from the fitting equation [15] as follows.
ρ=
(1)
where M
0
represents the molecular weight of H
2
O, V
m
and V
0
denote the partial molar volume of
the dry Nafion membrane and H
2
O. V
m
and V
0
could be calculated from V
m
= EW/ρ
m
and V
0
= M
0
/ρ
0
,
where ρ
m
is the density of dry Nafion membrane (2.05 g/cm
3
) and ρ
0
is the density of H
2
O. When the
hydration level increases from 0 to 14, experimental density of Nafion decreases from 2.05 g cm
-3
to
1.71 g cm
−3
, while the simulated values changes from 2.07 g cm
−3
to 1.74 g cm
−3
. Meanwhile,
SPEEK share the same tendency that the simulated density decreases with increasing hydration level.
Moreover, the simulated densities of SPEEK are well coincident with literature [16-18]. It could be
concluded that the simulated values of Nafion and SPEEK are accordant with experimental data very
well. It validates the accuracy of the molecular simulation performed in this study.
3.3. Hydrophilic clusters of hydrated Nafion and SPEEK systems
Radial distribution function of S-S for Nafion and SPEEK system presented are shown in Figure 4.
Both radial distribution functions exhibit a clear first peak at about 4.5Å. Nafion exhibits a much
more intensive peak than that of SPEEK. It proves that the agglomeration of hydrophilic domains,
represented by sulfonate groups, in SPEEK is worse than that in Nafion matrix, suggesting smaller
hydrophilic clusters and lower phase separation morphology in SPEEK. This is consistent with TEM
observations.
Hydrophilic cluster morphology of Nafion and SPEEK membranes with different hydration level
were explored by MD simulation. As typical representatives of hydrophilic groups, -SO
3
-
(or -SO
3
H
when hydration value=0), H
2
O and H
3
O
+
were carefully investigated and are presented in Figure 5. A
lower degree of phase separation of SPEEK, compared with that of Nafion, is observed, especially at
low water content. Moreover, the cluster sizes of SPEEK are smaller than that of Nafion for each
hydration level. This might be caused by the lower electronegativity of H than that F and higher
steric hindrance of SPEEK backbone. With increasing hydration level, H
2
O content increases
proportionally. Consequently, the hydrophilic clusters, composed of -SO
3
-
, H
2
O and H
3
O
+
, become
expanded and connected, resulting in wide and connected proton conducting channels.
Figure 3. Experimental and simulated density of
Nafion at different hydration level.
Figure 4. Radial distribution functions of S-
S pair for Nafion and SPEEK system (λ=0).
Morphology and Diffusion Behaviour of Nafion and SPEEK
555
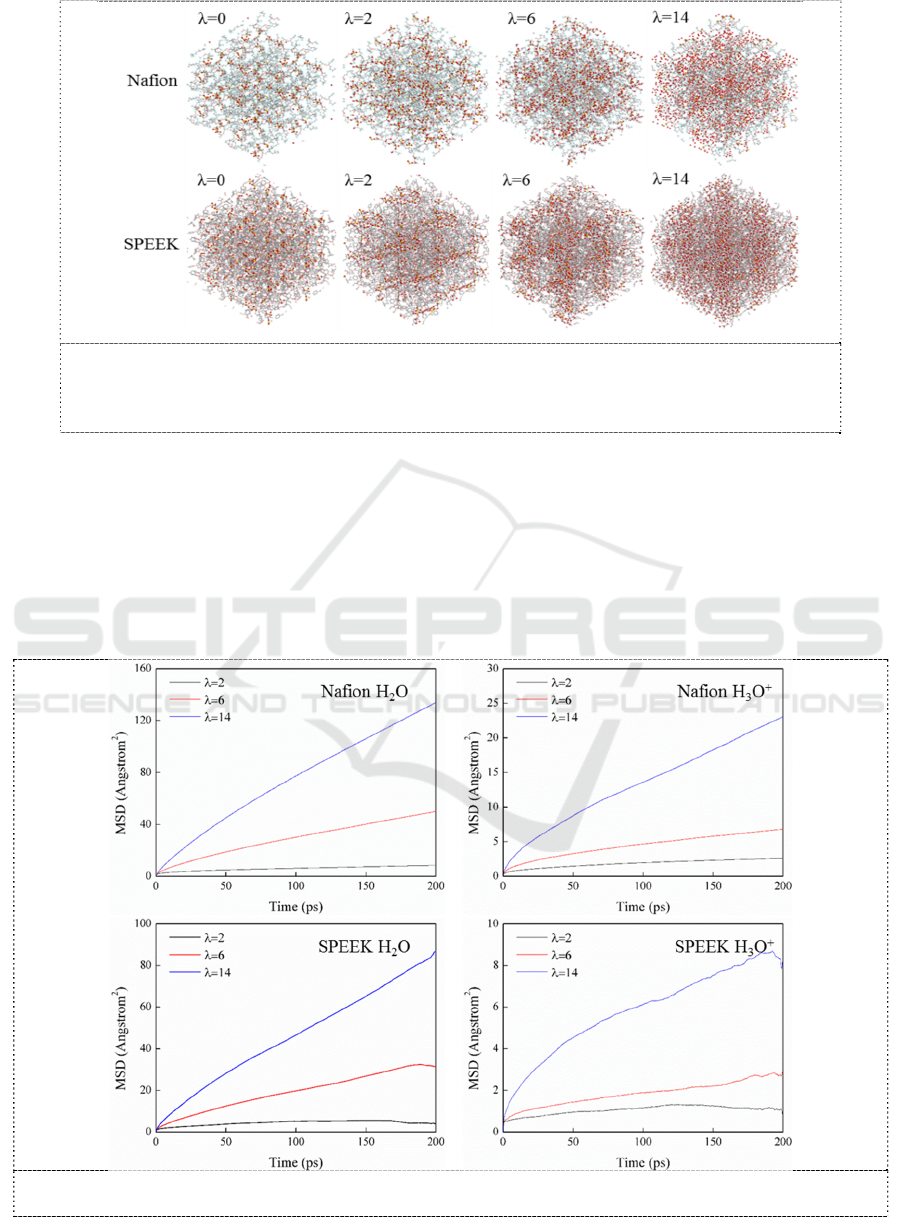
Figure 5. Final snapshots of -SO
3
-
, H
2
O and H
3
O
+
in Nafion and SPEEK matrix at
300 K. Red balls, oxygen in -SO
3
-
, H
2
O and H
3
O
+
; white balls, hydrogen in H
2
O and
H
3
O
+
; Yellow balls, sulfur in -SO
3
-
; other atoms were set in line mode.
3.4. Diffusion properties of Nafion and SPEEK
To investigate the effects of such cluster morphology on the mobility of H
2
O and H
3
O
+
, mean square
displacement (MSD) analysis was performed at 300 K according to equation (2) [19],
=
〈
0
〉
(2)
where r
i
(t) and r
i
(0) are the coordinates of atom i at specific time t=t and t=0, and the bracket
represents the ensemble average. Figure 6 exhibits the mean square displacements of H
2
O and H
3
O
+
in Nafion and SPEEK matrix at 300 K.
Figure 6. Mean square displacements of H
2
O and H
3
O
+
in Nafion and SPEEK matrix at 300 K.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
556

With the increasing of hydration level, the MSD of H
2
O and H
3
O
+
increase gradually. This will
lead to an increase in proton conductivity. In comparison with Nafion, SPEEK presents lower MSD
of H
2
O and H
3
O
+
, suggesting a lower proton transfer. This could be attributed to the worse phase
separation morphology in SPEEK than that of Nafion.
4. Conclusions
As one of the promising alternatives for Nafion, SPEEK possesses good chemical stability and
thermal stability, low cost, high mechanical stability, but poor hydrophilic cluster morphology and
insufficient proton conductivity. In this study, MD was performed to investigate the hydrophilic
cluster morphology and diffusion behavior of Nafion and SPEEK. SPEEK shows poorer phase
separation and much smaller hydrophilic channels especially at low hydration levels. This might be
caused by the lower electronegativity of H than that F and higher steric hindrance of SPEEK
backbone. Consequently, much lower mobility of H
2
O and H
3
O
+
are revealed for SPEEK. With
increasing hydration levels, the hydrophilic clusters are developed to be wide and connected proton
conducting channels, and H
3
O
+
mobility is enhanced as well. Therefore, a higher proton transfer is
expected at higher relative humidity.
Acknowledgment
The authors thank the financial support of the Natural Science Foundation of Hebei Province (Grant
no. B2018502046) and the Fundamental Research Funds for the Central Universities (Grant no.
2016MS110 and 2016MS111).
References
[1] Mader J A and Benicewicz B C 2010 Macromolecules 43 6706–15
[2] Xie X, Chen S, Ding W, Nie Y and Wei Z 2013 Chem. Commun. 49 10112-4
[3] Park M J and Kim S Y 2013 J. Polym. Sci. B: Polym. Phys. 51 481–493
[4] Park C H, Lee C H, Sohn J Y, Park H B, Guiver M D and Lee Y M 2010 J. Phys. Chem. B 114
12036–45
[5] Du L, Yan X, He G, Wu X, Hu Z and Wang Y 2012 Int. J. Hydrogen Energy 37 11853–61
[6] Qiu X, Dong T, Ueda M, Zhang X and Wang L 2017 J. Membrane Sci. 524 663-672
[7] Wang R, Yan X, Wu X, He G, Du L, Hu Z and Tan M 2014 J. Polym. Sci. Part B: Polym.
Phys. 52 1107-17
[8] O’Dea J R, Economou N J and Buratto S K 2013 Macromolecules 46 2267-74
[9] Dai J, Teng X, Song Y and Ren J 2017 J. Membr. Sci. 522 56-67
[10] Rao Z, Zheng C and Geng F 2018 Computational Materials Science 142 122-8.
[11] Tai C, Chen C and Liu C 2017 Int. J. Hydrogen Energy 42 3981-6
[12] Kusoglu A and Weber A Z 2017 Chem. Rev. 117, 987-1104
[13] Bahlakeh G, Nikazar M and Mahdi M H 2013 J. Membr. Sc 429
, 384-95
[14] Wang R, Wu X, Yan X, He G and Hu Z 2015 J. Membr. Sci. 479 46–54
[15] Weber A Z and Newman J 2004 J. Electrochem. Soc. 151(2) A311-25
[16] Bahlakeh G, Nikazar M, Hafezi M J, Dashtimoghadam E and Hasani-Sadrabadi M M 2012 Int.
J. Hydrogen Energy 37 10256-64
[17] Mahajan C V and Ganesan V 2010 J. Phys. Chem. B 114 8357-66
[18] Mahajan C V and Ganesan V 2013 J. Phys. Chem. B 117 5315-29
[19] Zhang W, Yue P L and Gao P 2011 Langmuir 27 9520-7
Morphology and Diffusion Behaviour of Nafion and SPEEK
557
