
Oxidation of MgF
2
Particles in SF
6
/Air Atmosphere at High
Temperatures
H K Chen
*
, L Chang and Y Y Jie
Baoji University of Arts and Science, Baoji 721013, PR China
Corresponding author and e-mail: H K Chen, hk7115@yahoo.com
Abstract. High temperature o xidation of MgF
2
particles in the atmosphere of air containing
different concentrations of SF
6
were studied by using XRD and EDS. The results show that
the oxidation of MgF
2
in SF
6
/air at mosphere was mainly related to the concentration of SF
6
,
temperature and reaction time. In the at mosphere of air containing higher SF
6
concentration,
MgF
2
underwent a very weak oxidation. With the decrease of SF
6
concentration, the increase
of temperature and the prolongation of reaction time, the degree of MgF
2
oxidation increased.
The particle size o f MgF
2
and the mixed gas flow have little effect on the high temperature
oxidation of MgF
2
. The results can provide a theoretical basis for the study of the protection
mechanism of SF
6
gas on magnesium and its alloy melt.
1. Introduction
Magnesium and its alloy are being widely used in the automotive industry, aerospace industry, 3C
products and other fields due to their excellent properties such as low density and high specific
strength. However, magnesium is a very active element. It has extremely high affinity for oxygen and
high vapor pressure, which causes molten magnesium to rapidly oxidize in air. Therefore, it is
necessary to take measures to prevent the melt from oxidizing or burning in the process of melting
and casting operations. Many methods have been investigated to inhibit the oxidation of molten
magnesium and its alloy in air. Among them, the protection method of SF
6
gas is considered to be the
most effective method [1]. The protection of SF
6
gas for magnesium and its alloy melt is based on a
dense MgF
2
and MgO composite protective film on the surface of the melt. MgF
2
, as a major
component of the film, plays a key role in the protection of magnesium melt against its combustion
by SF
6
and other fluorine-containing gases [2-4].
It is generally believed that MgF
2
is a stable compound under normal circumstances. However,
some studies have found that MgF
2
become less stable at high temperatures in air and it has a
tendency to change to MgO [5, 6]. In a previous study, we also found this phenomenon [7,8]. In
SF
6
/air atmosphere, whether MgF
2
will oxidize at high temperatures and what rule it follows if the
oxidation occurs, these problems are unclear. In view of the important role of MgF
2
in the protection
of magnesium melt by SF
6
, it is necessary to study the high temperature oxidation behavior of MgF
2
in the atmosphere. In this work, the oxidation characteristics of MgF
2
particles in SF
6
/air atmosphere
at high temperatures were studied. The effects of the concentration of SF
6
, temperature, and reaction
time on the oxidation characteristics were investigated. The purpose of this paper is to elucidate the
Chen, H., Chang, L. and Jie, Y.
Oxidation of MgF2 Particles in SF6/Air Atmosphere at High Temperatures.
In Proceedings of the International Workshop on Materials, Chemistr y and Engineering (IWMCE 2018), pages 507-512
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
507
conditions and laws of oxidation reaction of MgF
2
in SF
6
/air atmosphere, which will provide a
theoretical basis for the optimization of protection conditions of SF
6
gas for magnesium melt.
2. Experimental
The main raw materials used in the tests were high purity MgF
2
powder and SF
6
gas. The
composition of MgF
2
powder (wt%) is MgF
2
99.99, Na 0.001, Fe 0.001, Si 0.002, Ca 0.002, SO
4
2+
0.002, H
2
O 0.001, Pb less than 0.001. The composition of SF
6
gas (wt%) is SF
6
≥99.999,
H
2
O≤0.0001.
The oxidation of MgF
2
powder was studied by means of a high-temperature test method. The tests
were carried out in a SK-G05123K tube furnace in the SF
6
/air atmosphere. The apparatus includes a
SF
6
and air supply device, a Φ110×420 mm tube furnace and a Φ50×1000 mm silica-glass tube with
an alumina crucible. In experiment, air and SF
6
were mixed in the required proportion and then
continuously fed into the silica-glass tube at 200 ml/min. After purging inside the silica-glass tube
using the gas mixture for at least 1 h, MgF
2
sample was placed in the crucible, and then heated to the
desired temperament at a rate of 8
o
C/min and hold for a certain time. After that, the sample was
cooled down to room temperature, and stored in a dryer for the X ray diffraction (XRD) analysis and
energy dispersive spectrometer (EDS) analyses.
XRD analysis of the oxidized MgF
2
samples was carried out on a Rigaku Ultima IV X-ray
diffractometer with a Cu-Kα source operated at 40 kV and 40 mA. The elemental composition of the
oxidized MgF
2
samples was investigated by an EDAX Genesis APEX energy dispersive spectroscopy
assembled in Quanta FEG 250 field emission scanning electron microscope.
3. Results and discussion
3.1. Effects of SF
6
concentration on MgF
2
oxidation
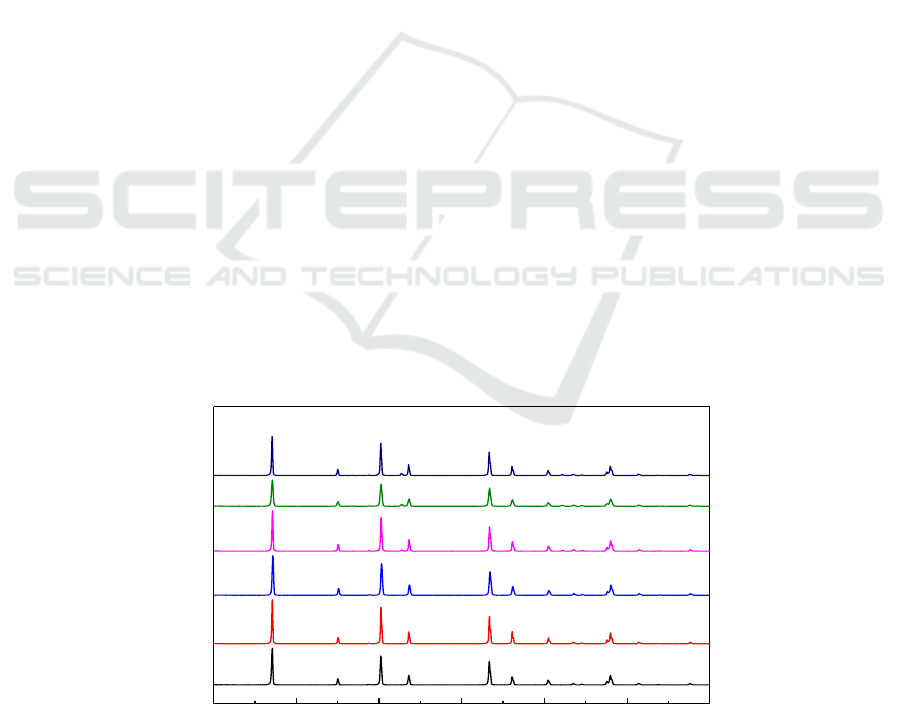
Figure 1 presents the XRD analysis results of MgF
2
samples oxidized in the atmospheres of air
containing different concentrations of SF
6
for 2 h at 1000
o
C. It can be seen that in the atmospheres of
air containing 1% SF
6
or 0.5% SF
6
, only the MgF
2
peak was detected. As the concentration of SF
6
was reduced to 0.1%, one new peak attributed to MgO occurred. When the concentration of SF
6
decreased from 0.05% to 0.01%, another MgO peak appeared outside the peak of SF
6
concentration
of 0.1%. The results indicate that with the decrease of SF
6
concentration, the oxidation degree of
MgF
2
increased.
20 30 40 50 60 70 80
■
▼
▼
■■
■
■
■
■
■
■
■
■
■
▼MgO ■ MgF
2
0.01% SF
6
0.05% SF
6
0.1% SF
6
0.5% SF
6
2θ (degree)
Intensity(a.u.)
1% SF
6
raw material
Figure1. XRD analysis results of MgF
2
samples oxidized in air containing different concentrations
of SF
6
at 1000
o
C for 2 h.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
508
The samples were also analyzed by EDS and the results are shown in Table 1. We can see from
the table that with the decrease of SF
6
concentration, the content of F element decreased and the
content of O element increased, which indicates that the oxidation degree of MgF
2
increased with the
decrease of SF
6
concentration. In addition, EDS detected a small amount of O element at SF
6
concentrations of 0.5% and 1%, indicating that the sample contained a small amount of MgO, but
XRD did not detect the presence of MgO. The reason might be that the content of MgO was lower
than the detection limit of XRD.
The reason for the increase in the oxidation degree of MgF
2
with decreasing SF
6
concentration is
as follows. In the mixed gas with high concentration of SF
6
, SF
6
will decompose to form more
reactive species like F
2
at high temperature. The resulting F
2
may react with MgO produced by the
oxidation reaction to form MgF
2
, which makes MgO actually not produced. In the mixed gas with
low SF
6
concentration, since the content of SF
6
in the mixed gas is very small, as is the case where
MgF
2
is exposed to high temperature air, MgF
2
would change into MgO by oxidation reaction [8].
Therefore, MgF
2
will undergo oxidation reaction in the mixed gas with low concentration of SF
6
.
Table 1. EDS analysis results of MgF
2
samples oxidized in air containing different concentrations of
SF
6
at 1000
o
C for 2 h (at%)
SF
6
concentration/%
F
Mg
O
1
62.42
36.44
0.84
0.5
62.70
36.16
1.05
0.1
61.40
36.15
2.45
0.05
60.60
36.18
3.22
0.01
59.83
36.54
3.63
3.2. Effects of temperature on MgF
2
oxidation
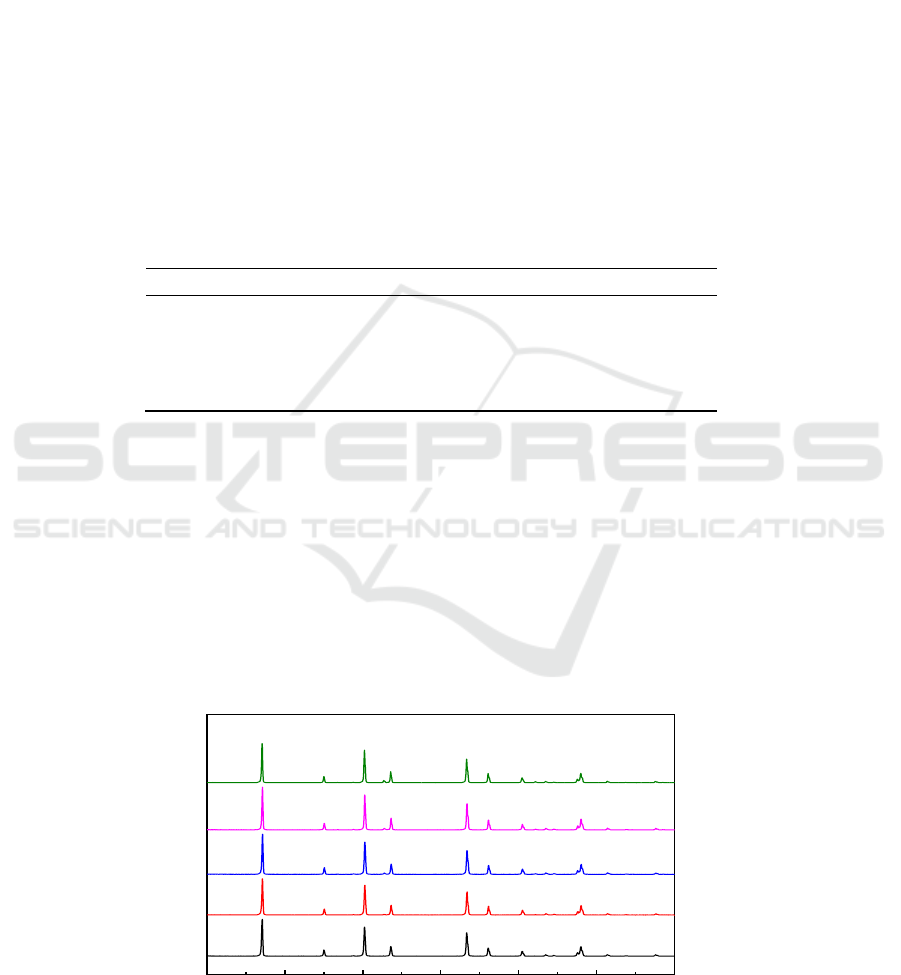
Figure 2 presents the XRD analysis results of MgF
2
samples after oxidation at different temperatures
in 0.01% SF
6
/air atmosphere for 2 h. As can be seen, when the temperature was 850
o
C, a very weak
MgO peak appeared. As the temperature increased from 900
o
C to 1000
o
C, another one MgO peak
appeared besides the one at 850
o
C. The results indicate that MgF
2
was oxidized and converted to
MgO at high temperature in the atmosphere, and the oxidation degree increased with the increase of
temperature. Table 2 shows the EDS results of MgF
2
samples after oxidation at the above condition.
The EDS results showed that as the temperature rose from 850
o
C to 1000
o
C, the content of F element
decreased, while the O element content increased. That is to say that the degree of oxidation of MgF
2
increased with the increase of temperature. These results are consistent with the results of XRD
analysis above.
20 30 40 50 60 70 80
▼▼
■
■
■
■
■
■
■
■
■
■
■
■
1000
o
C
950
o
C
900
o
C
850
o
C
Intensity(a.u.)
2θ (degree)
▼ MgO ■ MgF
2
raw material
Figure 2.XRD results of MgF
2
samples
oxidized in 0.01% SF
6
/air mixture at different temperatures
for 2 h.
Oxidation of MgF2 Particles in SF6/Air Atmosphere at High Temperatures
509
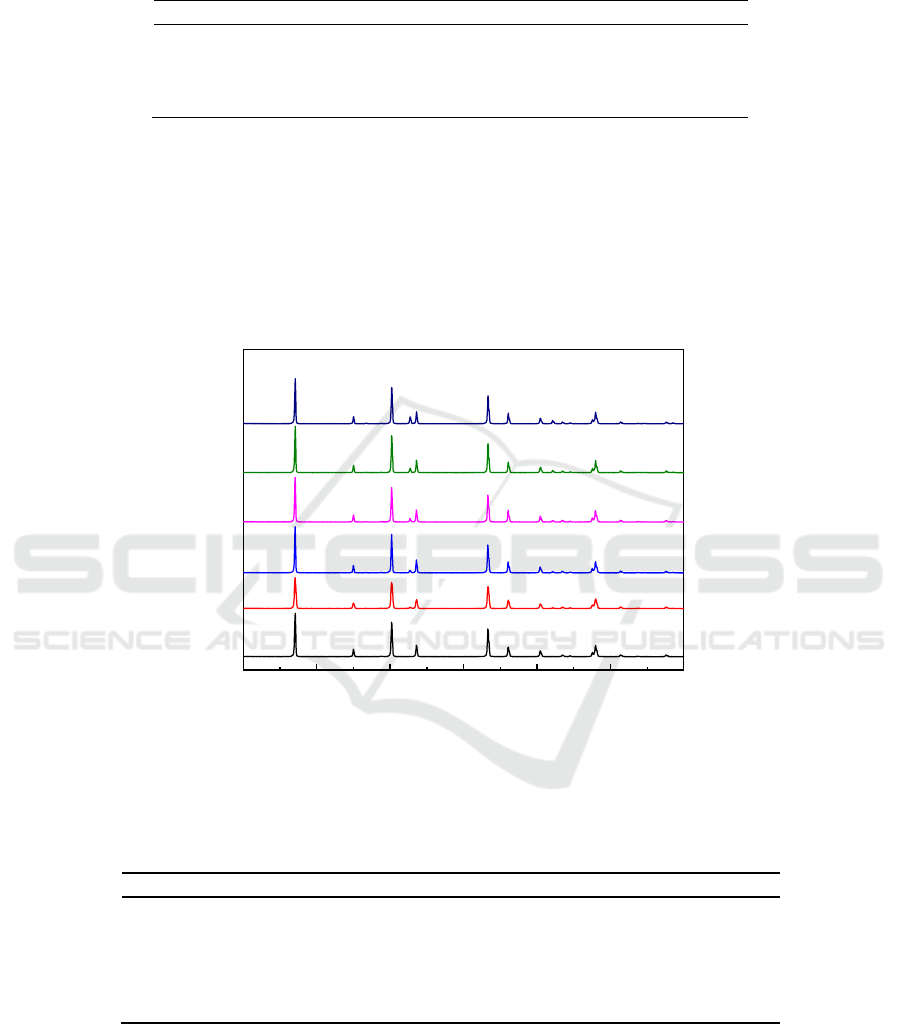
Table 2. EDS results of MgF
2
samples oxidized in 0.01% SF
6
/air mixture at different temperatures
for 2 h (at%)
Reaction temperature/
o
C
F
Mg
O
850
61.76
36.28
1.96
900
61.43
36.38
2.19
950
61.16
36.48
2.36
1000
59.83
36.54
3.63
3.3. Effects of reaction time on MgF
2
oxidation
Figure 3 shows the XRD analysis results of MgF
2
samples oxidized in 0.01% SF
6
/air atmosphere at
1000
o
C for different reaction times. It can be seen that the oxidation degree of MgF
2
increased with
the increase of reaction time. When the reaction time was 1 h, two weak MgO peaks appeared. As the
reaction time increased from 1 h to 5 h, the content of MgO increased and MgF
2
content decreased.
Table 3 shows the EDS analysis results of MgF
2
samples oxidized under the above conditions. The
EDS results indicated that the tendency of MgF
2
oxidation to MgO increased with the reaction time.
20 30 40 50 60 70 80
▼
▼
5 h
4 h
3 h
2 h
■
■
■
■
■
■
■
■
■
■
■
■
Intensity(a.u.)
2θ (degree)
▲MgO ■ MgF
2
1 h
raw material
Figure 3.XRD results of MgF
2
samples oxidized in 0.01% SF
6
/air atmosphere at 1000
o
C for
different reaction times.
Table 3. EDS results of MgF
2
samples oxidized in 0.01% SF
6
/air mixture at 1000
o
C for
different times (at%)
Reaction time/h
F
Mg
O
1
61.51
36.39
2.09
2
59.83
36.54
3.63
3
55.14
40.98
5.03
4
57.00
38.19
7.42
5
55.73
38.11
9.30
3.4. Effects of gas flow and particle size on MgF
2
oxidation
The XRD results of MgF
2
samples oxidized in 0.01% SF
6
/air mixed gas at 1000
o
C at different gas
flows and particle sizes for 2 h are shown in Figure 4. According to Figure 4, as the flow of mixed
gas and the particle size of the sample increased, the intensity of MgO peak was almost unchanged.
Table 4 shows the results of EDS analysis of MgF
2
samples oxidized under the same conditions. It
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
510
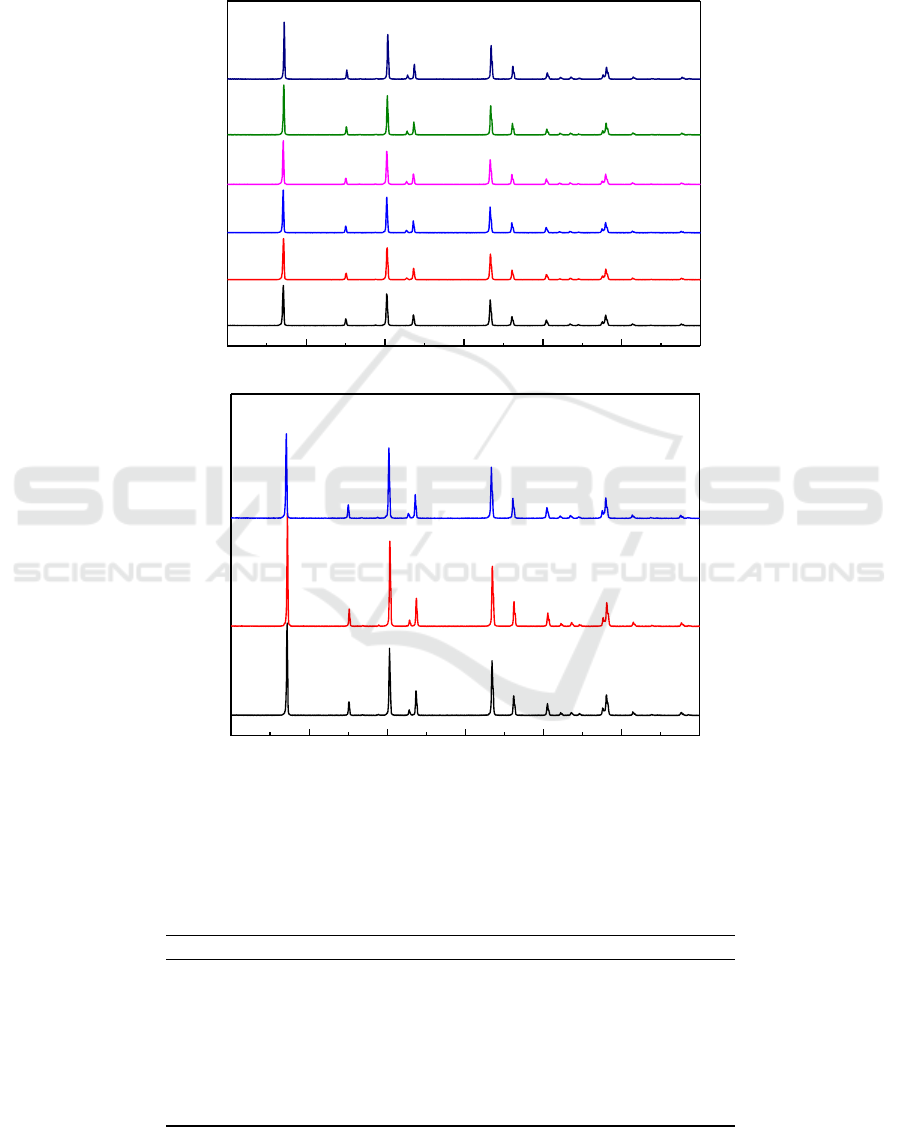
can be seen that oxygen content was almost unchanged, which means that the effect of particle size
and gas flow on the oxidation of MgF
2
are also very small. The reason may be that the flow of gas
was too high. In addition, it can be seen from the MgO peak in the XRD results by using the Scherrer
equation that MgO is a small particle.
20 30 40 50 60 70 80
▼
▼
500 ml min
-1
400 ml min
-1
300 ml min
-1
200 ml min
-1
▼MgO ■MgF
2
■
■
■
■
■
■
■
■
■
■
■
■
Intensity(a.u.)
2θ (degree)
raw material
100 ml min
-1
(a)
20 30 40 50 60 70 80
▼
▼
■
■
■
■
■
■
■
■
■
■
■
■
▼MgO ■MgF
2
200 mesh
100 mesh
2θ (degree)
Intensity(a.u.)
40 mesh
(b)
Figure 4.XRD results of MgF
2
samples oxidized in 0.01% SF
6
/air mixed gas at 1000
o
C for 2 h (a)
different flows, (b) different particle sizes.
Table 4.EDS results of MgF
2
samples oxidized at 1000
o
C in 0.01% SF
6
/air atmosphere for 2 h at
different gas flows and particle sizes (at%)
Gas flow/ml min
-1
Sieve/mesh
F
Mg
O
100
200
59.24
37.02
3.73
200
59.83
36.54
3.63
300
56.77
39.46
3.77
400
59.24
37.02
3.76
500
59.18
36.97
3.86
200
40
60.16
36.64
3.67
100
60.21
36.56
3.73
Oxidation of MgF2 Particles in SF6/Air Atmosphere at High Temperatures
511

4. Conclusions
The oxidation of MgF
2
particles in SF
6
/air atmosphere at high temperatures was studied. It was found
that the oxidation of MgF
2
in the atmosphere was mainly related to the concentration of SF
6
,
temperature and reaction time. With the decrease of SF
6
concentration, the increase of temperature
and the prolongation of reaction time, the degree of oxidation of MgF
2
increased. The particle size of
MgF
2
and the mixed gas flow had little effect on the high temperature oxidation of MgF
2
. The results
can provide a theoretical basis for the study of the protection mechanism of SF
6
gas on magnesium
and its alloy melt.
Acknowledgments
This work was supported by the National Natural Science Foundation of China under grant No.
51471003.
References
[1] Pang S, Wu G H, Sun M, Dai J C, Zhang Y and Ding W J 2011China Foundry 60 259–26
[2] Cashion S P, Ricketts N J and Hayes P C 2002 J. Light Met. 2 43–47
[3] Pettersen G, Øvrelid E, Tranell G and J Fenstad 2002 Mater. Sci. Eng. A 332 285–294
[4] Chen H K, Liu J R and Huang W D 2010 Corr. Sci. 52 3639–3645
[5] Xu Z Y, Sun D M, Li A X and Sun Z Q 2002 J. Chin. Ceram. Soc. 30 505–508
[6] Liu Y M, Zhao Z Y, Li A X, Yang C T, Han J Y and Sun Z Q 2002 J. Anhui Univ. (Nat. Sci.
Ed.) 26 46–50 (in Chinese)
[7] Chen H K, Jie Y Y and Chang L 2017 IOP Conf. Series: Mater. Sci. Eng. 170 012035
[8] Chen H K, Chang L and Jie Y Y 2017 Corr. Sci. 126 121–126
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
512
