
Selective Absorption of H
2
S from Gas Mixtures Contains
CO
2
into New Sterically Hindered Amine DTBP for Natural
Gas Purification
C Y Yang
1,2, *
, H G Chang
1,2
, J L He
1,2
, C R Wen
1,2
, H Yi
1,2
, C Y Tu
1,2
and S S
Qu
1,2
1
Research Institute of Natural Gas Technology, Petro China Southwest Oil & Gas
field Company, Chengdu, 610213, China
2
National Energy R&D Center of High Sulfur Gas Exploitation, Chengdu, 610213,
China
Corresponding author’s e-mail address: C Y Yang, yangchaoyue@petrochina.com.cn
Abstract. In this work, an experimental investigation for selective absorption of H
2
S from
gas mixtures into aqueous solutions of new sterically hindered amine for natural gas
purification at atmospheric pressure was performed. The new sterically hindered amine with
two tertiary butyl alkyls respectively attaches to two N atoms was called DTBP synthesized
and characterized by IR and 1HNMR. The effect of concentration and temperature of
solutions on absorption performance were investigated by means of the selectivity factor and
sour gas load. The performance of simultaneous absorption of CO
2
and H
2
S in to the aqueous
solution of DTBP is compared with that of the aqueous solutions of MDEA and AMP. The
aqueous solution of DTBP has been found to be an efficient solvent for selective H
2
S
removal. The experimental results also testified the advantages of severe sterically hindered
amines (e.g., DTBP and AMP) over traditional amines in selective H
2
S absorption. This work
provides the results for sterically hindered amine to be extensively applied in the field of
selective H
2
S removal.
1. Introduction
Natural gas purification is a very important part in the natural gas industry. The removal of H
2
S and
CO
2
from industrial gas streams is a significant operation in natural gas processing, hydrogen
purifying, refinery off-gas treating and synthesis gas for ammonia and methanol manufacturing. The
exploited natural gas composition is very complicated, and most of which contain hydrogen sulfide
and carbon dioxide.
At present, one of the methods that the natural gas commonly used for purification is alcohol
amine method. Methyldiethanolamine (MDEA) has been widely used in production for its selectivity
of H
2
S, good desulfurated efficiency and not easy foaming [1].
However, the content of H
2
S and CO
2
are low in many gas fields, especially the content of CO
2
is
under standard of commodity natural gas in China (< 2%). But the proportion of CO
2
/H
2
S is high
(more than 10, even more than 100). In view of this kind of situation, it’s necessary to fully
strengthen the solvent selected absorption function in order to reduce energy consumption and
456
Yang, C., Chang, H., He, J., Wen, C., Yi, H., Tu, C. and Qu, S.
Selective Absorption of H2S from Gas Mixtures Contains CO2 into New Sterically Hindered Amine DTBP for Natural Gas Purification.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 456-465
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
Improve the quality of sour gas into the sulfur recovery unit on condition of ensure purification
degree of H
2
S.
Since the mid-80s of 20th century, lots of research on selective desulfurizing solvent has been
developed [2]. In this paper, sterically hindered amine which is a kind of selectivity desulfurizing
solvent is researched. Recently, Exxon Research and Engineering Company have developed a kind of
sterically hindered amine-based H
2
S-selective gas treating processes. The processes have been
commercially applied. They claimed that the new hindered amine-based processes would be
potentially attractive replacements for the existing selective H
2
S removal processes including MDEA
based and direct conversion processes [3].
Sterically hindered amine belong to the compounds that one or two replace groups attached to the
nitrogen atoms in their molecules, which can produce sterically hindrance effect of the new type of
organic amine [4]. The effect makes amino-group more chemical activation[5]. For example, 2-
amino-2-methyl-1-propanol(AMP), menthane diamine(MDA) and 2-piperidinealcohol(PE).
Since the sterically hindered amine was reported, many of the researches have been studied by
domestic and foreign scholars. Presented a process development work using hindered amine as the
promoter of hot carbonate solutions for simultaneous absorption of H
2
S and CO
2
Say et al. [6].
Numerically interpreted the simultaneous mass transfer of CO
2
and H
2
S into aqueous blends of
MDEA and DEA Rascol et al. [7]. The work of Mandal presented an experimental and theoretical
investigation of the simultaneous absorption of CO
2
and H
2
S into aqueous blends of AMP and DEA
[8]. Simulated selective H
2
S absorption in aqueous amine solutions using a rate-based mode Nadhir
et al. [9] and Markus et al. [10]. However, in the current literature, studies on a sterically hindered
amine, especially a severe hindered amine (e.g., AMP) for high selective removal of H
2
S from gas
streams containing CO
2
and H
2
S are rarely reported.
This work synthesized a kind of new sterically hindered amine with two tertiarybutyl alkyls
respectively attaches to two N atoms which called DTBP. H
2
S-selective absorption into the aqueous
solution of MDEA, AMP and DTBP were investigated. The experiments dealt with the system of
simultaneous absorption of tri-component gases (CO
2
, H
2
S, N
2
) into the aqueous solution of
absorbents (MDEA, AMP, DTBP) in a absorber at atmospheric pressure in this work. The effects of
temperature, amine concentration were studied. Furthermore, experimental data from the solution of
BTAP was compared with that from the aqueous solution of MDEA and AMP. Sterically hindered
amine selectivity of H
2
S(S) was determinated. The emphasis of this work focused on the selective
removal of H
2
S from gas mixtures containing both CO
2
and H
2
S using the DTBP aqueous solution.
2. Theory
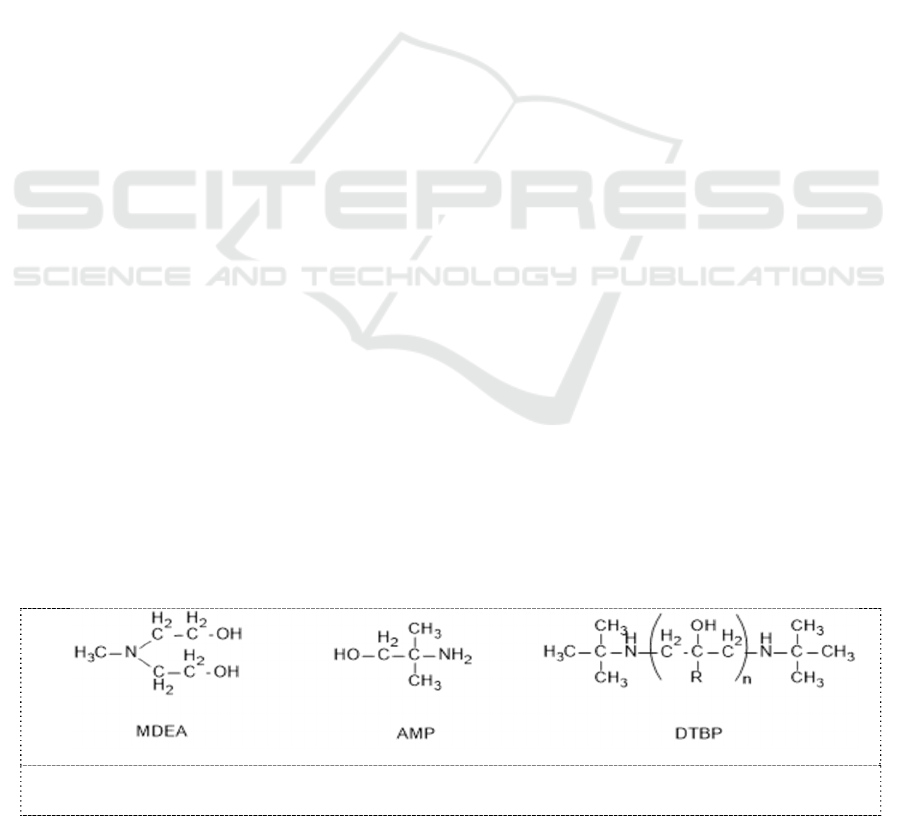
2.1. Characteristics of molecular structure of sterically hindered amines
Amines are commonly subdivided into primary, secondary and tertiary amines according to the
number of alkyl groups attached to N atom in the molecule of amines. MDEA belongs to tertiary
amines, AMP belongs to primary amine and DTBP belong to secondary amines, and their molecule
structures are shown in Figure 1.
Figure 1. Molecule structure of MDEA, AMP and DTBP.
Selective Absorption of H2S from Gas Mixtures Contains CO2 into New Sterically Hindered Amine DTBP for Natural Gas Purification
457

A nonlinear alkyl group, tertiary butyl alkyl group, attaches to N atom in AMP molecule, and two
tertiary butyl alkyls respectively attaches to two N atoms in DTBP molecule. R stands for H atom or
methyl molecule and n is greater than or equal to 1. It occupies bulky volume in space, and hinders
activity of amino-group. The hindrance of nonlinear alkyl group gives amino-group of AMP and
DTBP rise to higher chemical activation than that of non-hindered amines. The chemical activation is
the so-called sterically hindered effect.
2.2. Reaction mechanism
The reaction mechanism for systems involving H
2
S, MDEA, AMP, DTBP is as follows:
H
S(g) ↔ H
S(l) H
2
S phase change (1)
2H
O↔OH
+H
O
dissociation of H
2
O (2)
H
S+H
O↔H
O
+HS
dissociation of H
2
(3)
HS
+H
O↔H
O
+S
dissociation of HS
-
(4)
MDEAH
+H
O↔H
O
+MDEA (5)
AMPH
+H
O↔H
O
+AMP (6)
DTBPH
+H
O↔H
O
+DTBP (7)
The reaction rate constants k values of Eq. (5), (6) and (7) are more than 10
9
L/(mol·s) (25°C)[11].
These reactions are completed instantaneously. according to the theory of double film of gas-liquid
mass transfer, everywhere in the liquid phase as well as the interfacial liquid film, H
2
S-amine
equilibriums exist always [12].
But the reaction of alcohol amine and carbon dioxide have a very different situation.
Carbon dioxide reaction with AMP and DTBP is as follows:
CO
+H
O+RNH
=RNH
+HCO
(8)
−CH
−OH+OH
+CO
= COCOO
+H
O (9)
CO
+R
R
NH + H
O=R
R
NH
+HCO
(10)
CO
+2R
R
NH = R
R
NH
+RNHCOO
(11)
The overall reaction is
CO
+R
R
NH + H
O=R
R
NH
+HCO
(12)
For the sterically hindered amine (such as AMP and DTBP), its carbamate is very unstable and
the reaction mechanism indicates that the ultimate product of the reaction is bicarbonate.
For the MDEA, because it is a kind of tertiary amine, CO
2
cannot directly react with MDEA to
form carbamate, but with H
2
O to form bicarbonate. Representative reactions are following:
CO
+H
O=H
+HCO
(13)
R
R
R
N+H
=R
R
R
NH
(14)
The overall reaction is
CO
+H
O+R
R
R
N=R
R
R
NH
+HCO
(15)
Comparing Eq. (12) with Eq. (15), it shows that the ultimate products of MDEA, AMP and DTBP
reaction with CO
2
are all bicarbonate. The reaction rate constants k values of Eq. (12) and (15) are
less than 7 L/(mol·s) (25°C) [13]. The liquid-film is dominant resistance of CO
2
mass transfer [14].
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
458
This shows that the reaction of H
2
S and CO
2
are different in kinetics. This is the theoretical
foundation of severe hindered amine (such as AMP and DTBP) can using as the absorbent to attain
higher H
2
S selectivity.
2.3. Evaluation of absorption performance
L is sour gas load. The value of
and
are calculated by following equation:
=
=
(17)
S is selectivity factor of sterically hindered amine. Selectivity of amine solvents for H
2
S may be
defined as the tendency for the ratio of H
2
S to CO
2
contents to be larger in the liquid phase than it is
in the gas phase. Selectivity factor is used as a yardstick for the H
2
S selectivity [15]. The value of
selectivity factor equates the ratio of H
2
S/CO
2
in liquid-phase to H
2
S/CO
2
in gas-phase and is
expressed as:
=
/
/
(18)
Where x is mole fraction of component i in the liquid bulk. y is mole fraction of component i in
the gas bulk.
3. Experimental
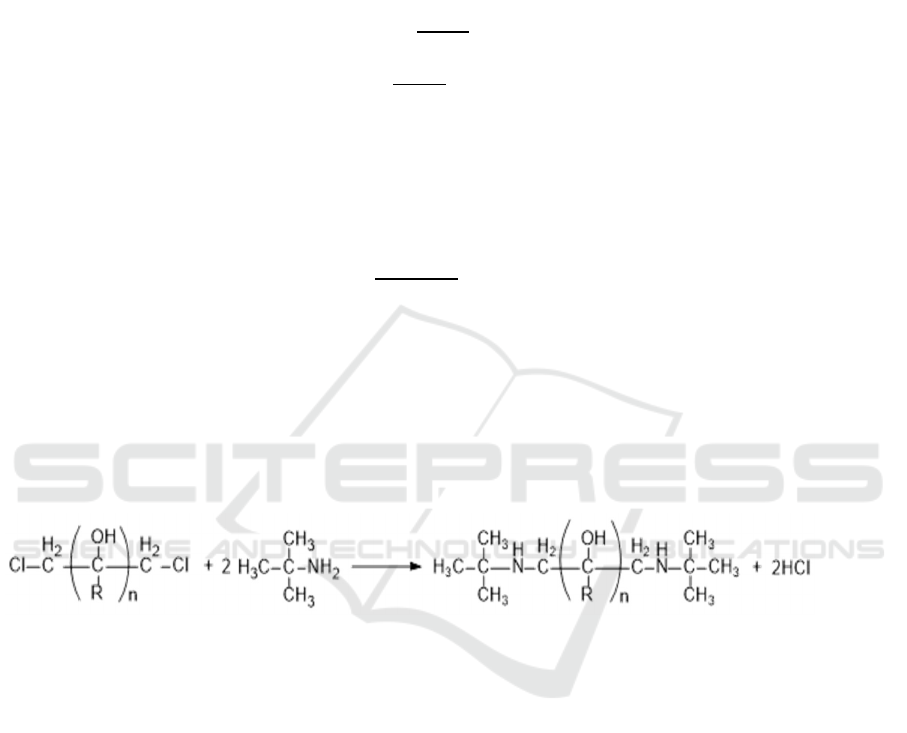
3.1. Synthesis experiments of DTBP
Reaction equation is given as:
(19)
Tert-butylamine reacted with chlorinated alcohol in ratio of 4:1, absolute ethyl alcohol 50% from
the total content of reactants was added as an azeotropic solvent. The mixture was heated at 120°C
with continuous stirring for 4 hours. Poured out after cooling, add a certain amount of NaOH, reflux
reaction under 70 °C for 50 min. Keep the filtrate after the suction filter, then vacuum distillation and
cooling crystallization.
The chemical structure was confirmed by the infrared absorption spectroscopy (FTIR) and nuclear
magnetic resonance (NMR) test. The results are shown in figure 2 and figure 3.
(16)
Selective Absorption of H2S from Gas Mixtures Contains CO2 into New Sterically Hindered Amine DTBP for Natural Gas Purification
459
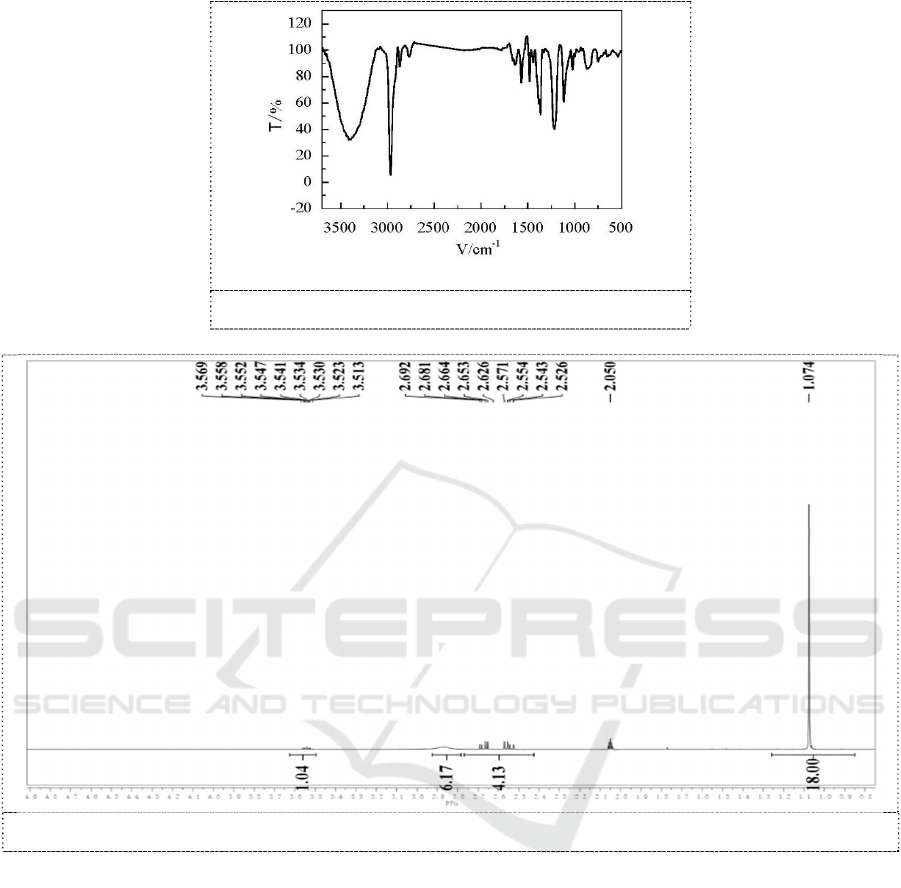
Figure 2 The infrared spectra of DTB
P
.
Figure 3 The Nuclear magnetic resonance spectra of DTB.
3.2. The experiment of H
2
S selective evaluation of MDEA, AMP and DTBP
MDEA(clear colorless liquid with a purity of ≥ 99 mass %) and AMP (clear colorless liquid with a
purity of ≥ 99 mass %) were supplied by Shanghai Aladdin biochemical Polytron Technologies Inc.
DTBP (clear colorless liquid with a purity of ≥ 99 mass %) is for this work synthesis. The gas
cylinder was supplied by Chemical Gas Company with the mole fraction purity of 10% CO
2
, 1%H
2
S
and 89% N
2
. Distilled H
2
O was produced using Europe ultra water equipment.
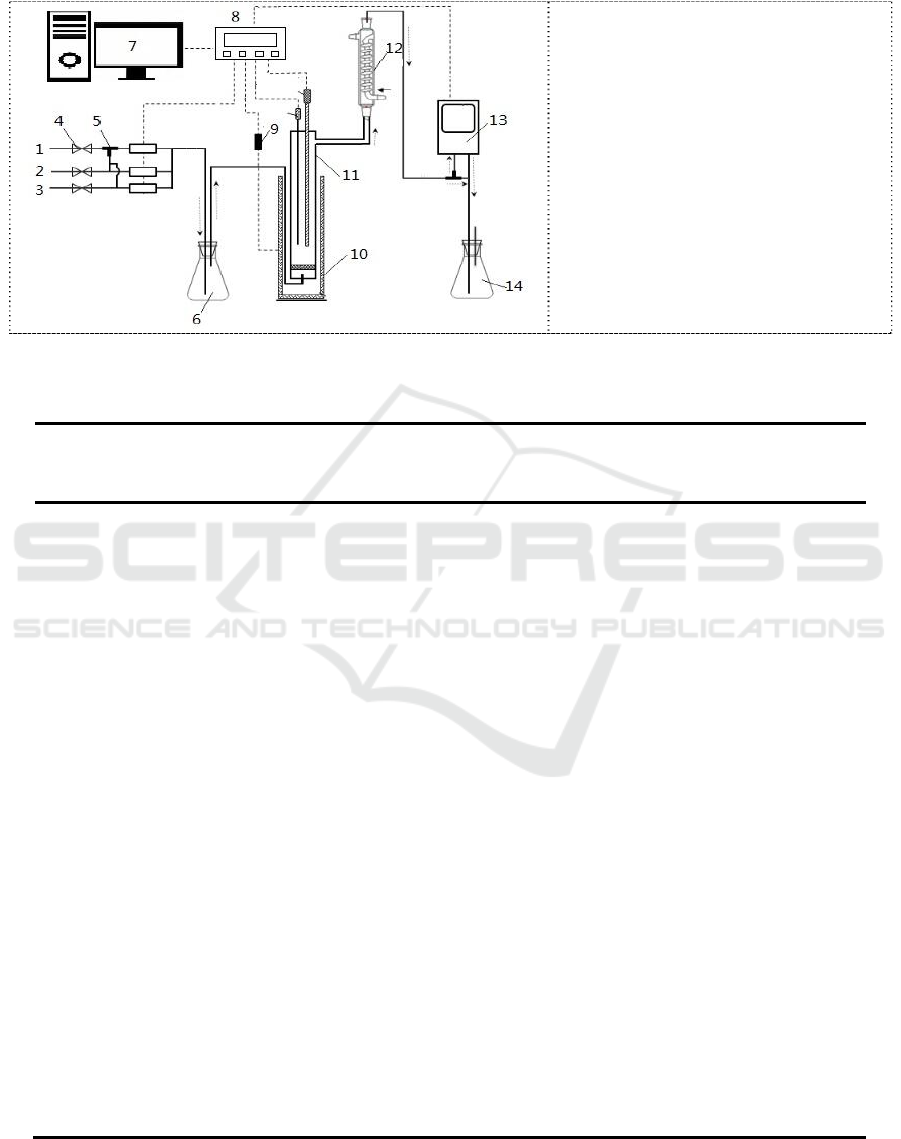
As shown in figure 4, the absorption/desorption apparatus was used to determine the selective
absorption of H
2
S from gas mixtures into solution of MDEA, AMP and DTBP for the systems
studied.
4. Results and discussions
This paper investigated the acid gas which has high carbon sulfur ratio (
:
=10:1). The
desulfurization performance of amine liquid (MDEA, DTBP, AMP) which a concentration of 10, 20,
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
460
30, 40, 50% have been tested at 20 to 60°C and compared with the same conditions of MDEA
solution. The experimental results are shown in table 1 to table 3.
Figure 4. Schematic diagram of the
experimental setup: 1, H
2
S input
unit; 2, CO
2
input unit; 3, N
2
input
unit; 4, cut-off valve; 5, three-way
valve; 6, buffer tank; 7, Record and
control unit; 8, Data acquisition
unit; 9, temperature sensor; 10,
micro controlled heating furnace;
11, absorption/desorption reactor;
12, condenser pipe; 13, gas
analyzer; 14, alkali liquor
absorption tank..
Table 1. The results of different concentrations of MDEA solution absorption acid gas at different
temperature
T
(
°C)
w(MDE
A)
(%)
(g/L)
( g/L )
(%)
(%)
L S
20 10 0.1049 0.2558 1.0061 9.9886 0.0637 0.1552 0.2188 4.07
20 20 0.1978 0.4907 1.0018 10.0041 0.0600 0.1488 0.2088 4.03
20 30 0.2552 0.6472 0.9957 9.9926 0.0516 0.1309 0.1825 3.96
20 40 0.3390 0.8613 0.9986 9.9980 0.0514 0.1306 0.1820 3.94
20 50 0.4329 1.1246 1.0002 10.0021 0.0525 0.1364 0.1890 3.85
30 10 0.1023 0.2559 0.9977 10.0018 0.0621 0.1553 0.2173 4.01
30 20 0.1831 0.4565 1.0027 9.9980 0.0555 0.1385 0.1940 4.00
30 30 0.2542 0.6364 0.9993 9.9975 0.0514 0.1287 0.1801 4.00
30 40 0.3188 0.8122 0.9982 10.0015 0.0483 0.1232 0.1715 3.93
30 50 0.4013 1.0259 1.0037 9.9993 0.0487 0.1245 0.1732 3.90
40 10 0.0939 0.2357 0.9977 10.0018 0.0570 0.1430 0.2000 3.99
40 20 0.1766 0.4504 1.0027 9.9980 0.0536 0.1366 0.1902 3.91
40 30 0.2420 0.6315 0.9993 9.9975 0.0489 0.1277 0.1766 3.83
40 40 0.3058 0.7883 0.9982 10.0015 0.0464 0.1196 0.1659 3.89
40 50 0.3565 0.9407 1.0037 9.9993 0.0433 0.1141 0.1574 3.78
50 10 0.0807 0.2031 1.0004 9.9956 0.0490 0.1232 0.1722 3.97
50 20 0.1530 0.3974 0.9963 10.0020 0.0464 0.1205 0.1669 3.87
50 30 0.2347 0.6081 0.9973 10.0042 0.0475 0.1230 0.1704 3.87
50 40 0.2723 0.6959 0.9990 9.9958 0.0413 0.1056 0.1468 3.91
50 50 0.3216 0.8386 0.9991 9.9999 0.0390 0.1017 0.1408 3.84
60 10 0.0758 0.1953 0.9984 10.0037 0.0460 0.1185 0.1645 3.89
60 20 0.1243 0.3253 0.9969 10.0042 0.0377 0.0987 0.1364 3.84
60 30 0.1774 0.4610 1.0010 9.9987 0.0359 0.0932 0.1291 3.84
60 40 0.2347 0.6221 1.0037 10.0038 0.0356 0.0943 0.1299 3.76
60 50 0.2760 0.7453 0.9986 10.0004 0.0335 0.0904 0.1239 3.71
Selective Absorption of H2S from Gas Mixtures Contains CO2 into New Sterically Hindered Amine DTBP for Natural Gas Purification
461
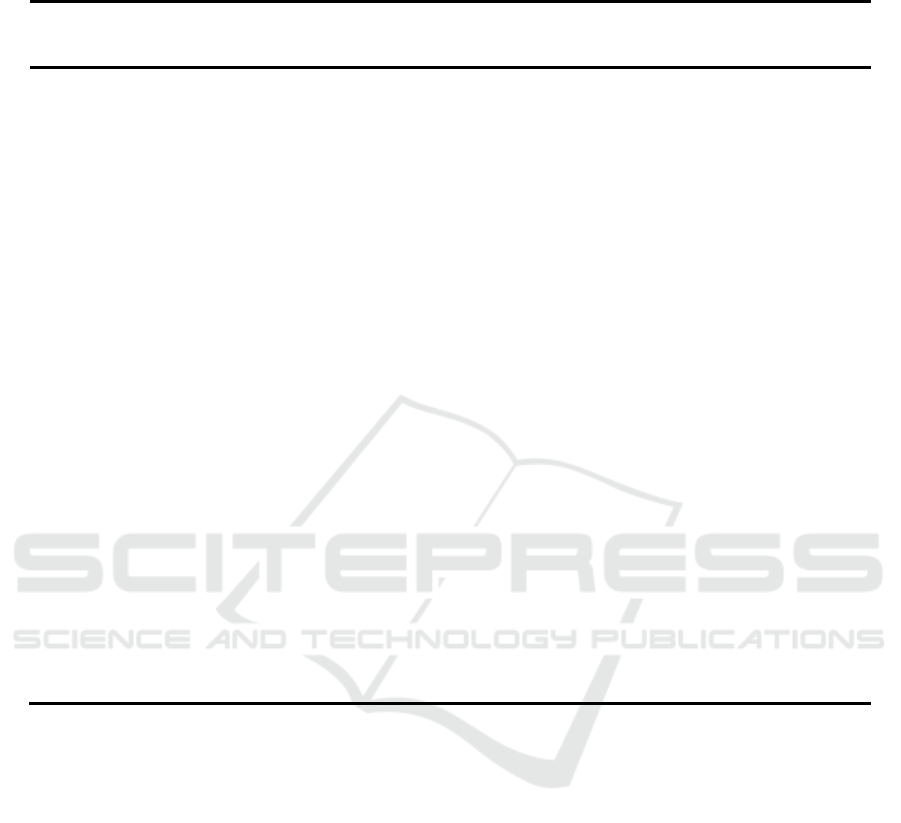
Table 2. The results of different concentrations of AMP solution absorption acid gas at different
temperature
From table 1to table 3 it can be seen that MDEA, DTBP, AMP of H
2
S and CO
2
absorption load is
reduced as the temperature increases, and the total acid gas load is reduced; with the increase of
concentration of amine liquid, acid gas load is also decreased.
Analysis the load of H
2
S, CO
2
and total acid gas load of MDEA, AMP and DTBP under different
concentration at 40°C. The results are shown in Figure 5.
From figure 5 it can be seen that as the concentration of amine increased, the content of
,
and
L kept less decreased.
(DTBP)>
(AMP)>
(MDEA),
(MDEA)>
(DTBP)>
(AMP) and L(DTBP)>L(AMP)>L(MDEA) at amine solution
concentration of 10%~40% at 40°C. Meanwhile, The same results can be obtained at 20~60°C. Under the
same concentration and temperature, H
2
S load of AMP and DTBP are greater than the MDEA,
(DTBP) is the biggest of the three amine and
(DTBP) is about 3 times than
(MDEA). But
(MDEA) is the biggest of the three amine,
(DTBP) is closed to
(AMP) at 40°C.
T
(°C)
w(AMP)
(%)
(g/L)
(
g/L)
(%)
(%)
L S
20 10 0.1748 0.1511 0.9978 9.9989 0.1433 0.1237 0.2671 11.60
20 20 0.3314 0.2862 1.0017 9.9966 0.1358 0.1173 0.2531 11.56
20 30 0.4897 0.4237 1.0021 9.9956 0.1338 0.1158 0.2496 11.53
20 40 0.6347 0.5551 0.9957 10.0006 0.1301 0.1137 0.2438 11.49
20 50 0.8056 0.7011 1.0024 9.9984 0.1321 0.1149 0.2470 11.46
30 10 0.1636 0.1418 0.9982 9.9970 0.1341 0.1162 0.2503 11.55
30 20 0.3255 0.2813 0.9992 10.0040 0.1334 0.1153 0.2487 11.58
30 30 0.4567 0.3960 0.9985 10.0043 0.1248 0.1082 0.2329 11.56
30 40 0.5997 0.5270 1.0017 9.9968 0.1229 0.1080 0.2309 11.36
30 50 0.7462 0.6551 1.0041 10.0004 0.1223 0.1074 0.2297 11.35
40 10 0.1589 0.1377 0.9991 9.9963 0.1303 0.1129 0.2432 11.54
40 20 0.3005 0.2605 0.9969 10.0027 0.1232 0.1068 0.2300 11.57
40 30 0.4359 0.3789 0.9975 9.9991 0.1191 0.1035 0.2226 11.53
40 40 0.5806 0.5044 1.0040 9.9956 0.1190 0.1034 0.2224 11.46
40 50 0.7242 0.6311 1.0033 9.9964 0.1187 0.1035 0.2222 11.43
50 10 0.1503 0.1310 1.0027 9.9978 0.1231 0.1074 0.2305 11.43
50 20 0.2776 0.2442 0.9992 10.0030 0.1137 0.1001 0.2138 11.37
50 30 0.3997 0.3526 1.0000 9.9999 0.1092 0.0963 0.2055 11.34
50 40 0.5306 0.4691 0.9983 10.0025 0.1087 0.0961 0.2048 11.34
50 50 0.6741 0.5944 0.9989 9.9982 0.1105 0.0974 0.2080 11.35
60 10 0.1334 0.1173 1.0037 10.0019 0.1094 0.0961 0.2055 11.35
60 20 0.2501 0.2208 1.0019 9.9981 0.1025 0.0905 0.1930 11.30
60 30 0.3604 0.3198 0.9958 9.9986 0.0985 0.0874 0.1858 11.32
60 40 0.4803 0.4274 0.9995 10.0011 0.0984 0.0876 0.1860 11.24
60 50 0.5914 0.5278 1.0039 9.9993 0.0969 0.0865 0.1835 11.16
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
462
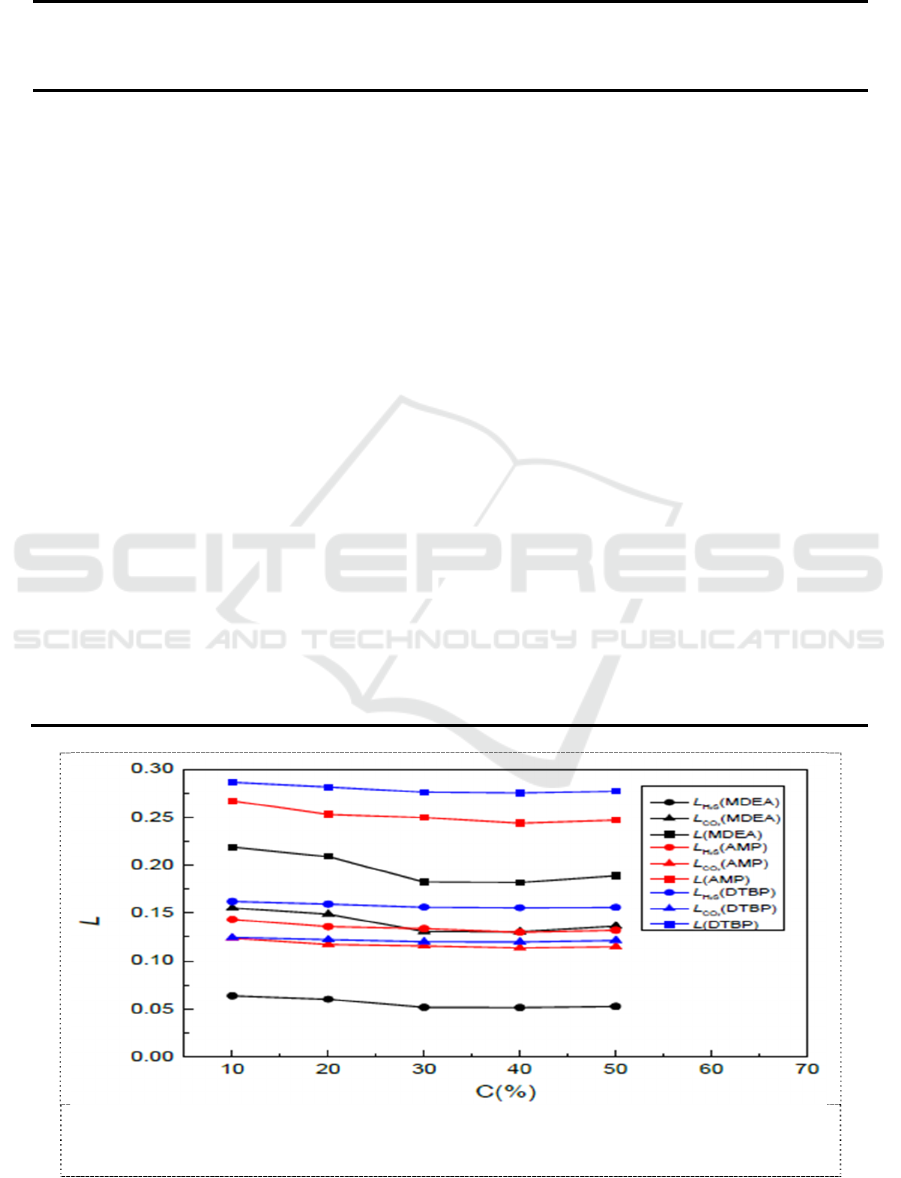
Table. 3 The results of different concentrations of DTBP solution absorption acid gas at different
temperature
Figure 5. The
,
and L of different concentrations of MDEA, AMP and
DTBP at 40
°C.
T
(
°C
)
w(DTBP
)
(%)
(g/L)
( g/L )
(%)
(%)
L S
20 10 0.1575 0.1210 0.9987 10.0028 0.1620 0.1244 0.2864 13.04
20 20 0.3100 0.2377 1.0040 10.0037 0.1594 0.1222 0.2816 12.99
20 30 0.4553 0.3504 1.0016 10.0043 0.1561 0.1201 0.2762 12.98
20 40 0.6048 0.4659 1.0040 9.9966 0.1555 0.1198 0.2753 12.92
20 50 0.7576 0.5895 0.9992 10.0028 0.1558 0.1213 0.2771 12.86
30 10 0.1430 0.1104 0.9993 9.9998 0.1471 0.1134 0.2606 12.98
30 20 0.2781 0.2145 1.0011 9.9979 0.1430 0.1103 0.2533 12.95
30 30 0.4068 0.3174 0.9964 10.0015 0.1395 0.1088 0.2483 12.87
30 40 0.5258 0.4101 1.0033 10.0044 0.1352 0.1054 0.2406 12.78
30 50 0.6354 0.5044 0.9975 9.9988 0.1307 0.1037 0.2344 12.63
40 10 0.1291 0.0994 0.9961 9.9971 0.1328 0.1022 0.2350 13.03
40 20 0.2420 0.1868 0.9977 9.9997 0.1244 0.0961 0.2205 12.98
40 30 0.3726 0.2872 1.0045 9.9991 0.1277 0.0984 0.2262 12.92
40 40 0.4807 0.3764 0.9971 10.0036 0.1236 0.0967 0.2203 12.81
40 50 0.6015 0.4716 1.0028 9.9992 0.1237 0.0970 0.2207 12.72
50 10 0.1137 0.0886 1.0010 10.0023 0.1169 0.0910 0.2079 12.83
50 20 0.2157 0.1687 0.9958 9.9970 0.1109 0.0868 0.1977 12.83
50 30 0.3123 0.2448 1.0040 10.0024 0.1070 0.0839 0.1909 12.71
50 40 0.4017 0.3163 0.9999 10.0008 0.1033 0.0813 0.1846 12.70
50 50 0.5001 0.3925 1.0025 10.0008 0.1028 0.0807 0.1836 12.71
60 10 0.0986 0.0780 0.9987 10.0037 0.1014 0.0802 0.1815 12.67
60 20 0.1941 0.1534 1.0028 9.9956 0.0998 0.0788 0.1786 12.62
60 30 0.2726 0.2165 1.0011 10.0000 0.0934 0.0742 0.1677 12.57
60 40 0.3392 0.2721 0.9985 9.9998 0.0872 0.0699 0.1571 12.49
60 50 0.4031 0.3251 0.9968 10.0045 0.0829 0.0669 0.1498 12.44
Selective Absorption of H2S from Gas Mixtures Contains CO2 into New Sterically Hindered Amine DTBP for Natural Gas Purification
463
Analysis the S of MDEA, AMP and DTBP under different concentration and temperature. The
results are shown in figure 6 and figure 7.
Figure 6. The S of different concentrations of
MDEA, AMP and DTBP at 40°C
Figure 7. The S of 40% MDEA, AMP and
DTBP at different temperature
Compared with the results in figure 6 and figure 7, the concentration and temperature had a much
smaller effect on S . S of AMP and DTBP are greater than MDEA, S of DTBP is the biggest of the
three amine.
5. Conclusions
In this work, a new type of sterically hindered amine DTBP was synthesized, and H
2
S selective
absorption from mixture gas into the aqueous solution of MDEA, AMP and DTBP have been
studied. The absorption performance of DTBP solution was compared with MDEA and AMP
solutions. The effect factors of temperature and the amine concentration on the performance were
investigated. The experimental results testified the advantages of sterically hindered amines (eg.,
AMP and DTBP) over traditional amines in selective H
2
S absorption.
MDEA, DTBP, AMP of H
2
S and CO
2
absorption load is reduced as the temperature increases,
and the total acid gas load is reduced; But with the increase of concentration of amine liquid, acid gas
load is increased. Low absorption temperature and high concentration of amine are in favor of
selection H
2
S absorption. But selective (S) was little affected by temperature and concentration. The
H
2
S selectivity order is S(DTBP)>S(AMP)>S(MDEA),
(DTBP)>
(AMP)>
(MDEA),
(MDEA)>
(DTBP)>
(AMP), L(DTBP)>L(AMP)>L(MDEA) under the same
absorption temperature and amine concentration. This suggests that the aqueous solution of DTBP is
an efficient solvent for selective H
2
S removal. The experimental results testified the advantages of
severe sterically hindered (e.g., DTBP and AMP) over traditional amines (MDEA) in selective H
2
S
absorption. Sterically hindered amine like DTBP can be extensively applied in the field of selective
H
2
S removal.
References
[1] Lu J G, Zheng Y F and He D L 2006 J. Sep. Purif . Technology 52 209
[2] Saha A K, Biswas A K and Bandyopadhyay S, 1999 J. Sep. Purif . Technology 15 101.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
464

[3] TAHERI M, MOHEBBI A, HASHEMIPOUR H, 2016 J. J Natural Gas Sci Eng 28 410
[4] Idris Z and Eimer D A 2014 J Ene. Procedia 51 247
[5] Sartori G and Savage D W 1983 J Ind. Eng. Chem 22 239.
[6] Say G R, Heinzelmann F J, Iyengar J N, Savage D W, Bisio A and Sartori G 1984 J Chem.
Eng. Prog 80 72.
[7] Rascol E, Meyer M and Prevost M 1996 P Comput. Chem. Eng 20 S1401
[8] Mandal B P and Bandyopadhyay S 2005 P Chem. Eng. Sci 60 6438.
[9] Al-Baghli N A, Pruess S A, Yesavage V F and Selim M F 2001 P Fluid. Phase. Equilibr 185
31
[10] Bolhàr-Nordenkampf M, Friedl A, Koss U and Tork T 2004 P Chem. Eng. Process 43 701
[11] Deshpande A S, Khomane B, Vaidya B K, Joshi R M, Harle A S and Kulkarni B D 2008 P
Nanoscale Res Lett 3 221.
[12] Kennedy N, Zhao Q B, Ma J, Chen S and Frear C 2015 J Sep. Purif . Technology 144 240
[13] Ko J and Li M H 2000 J Chem. Eng. Sci 55 4139
[14] Cornelissen A E 1980 J Tran. Ins. Chem. Eng 58
[15] Mandal B P, Biswas A K and Bandyopadhyay S 2004 J. Sep. Purif . Technology 35 191
Selective Absorption of H2S from Gas Mixtures Contains CO2 into New Sterically Hindered Amine DTBP for Natural Gas Purification
465
