
Effect of Surface Quality on Pitting Corrosion Behavior of
Aluminum Alloy 2A12
Z Li
1, 2, 3,*
, S L Lv
1
, Z E Liu
4
and W Zhang
1
1
National Key Laboratory of Science and Technology on UAV, Northwestern
Polytechnical University, Xi’an 710065, China
2
School of Aeronautics, Northwestern Polytechnical University, Xi'an 710072, China
3
School of Mechanical Engineering, Shaanxi Institute of Technology, Xi’an710300,
China
4
AVIC The First Aircraft Institute, Xi’an 710089, China
Corresponding author and e-mail: Z Li, limc@mail.nwpu.edu.cn
Abstract. Surface quality is one of the most important factors of corrosion damage in aircraft
structure. In this paper, quantitative method was used for surface quality, the surface
roughness was obtained by scanning the specimens with a scanner. Accelerated corrosion
experiments of aluminum alloy 2A12 were performed in the laboratory to analyze the effect
of surface quality on the occurrence and development of pitting corrosion. The results of the
experiments show that in the initial stage of the corrosion, a rough surface is more susceptible
to pitting corrosion and have a larger corrosion velocity. The corrosion velocity increases
with time. With the extension of time, the effect of surface quality on the corrosion behavior
tends to disappear.
1. Introduction
Aluminum alloy 2A12 is a high-strength aluminum-copper alloy, which has the advantages of low
density, high specific strength, and good weldability. It is widely used in aircraft and other aerospace
products. During the flight of an aircraft, corrosion may occur due to the corrosive effect of Cl‾ in the
atmosphere. Among various types of corrosion, pitting corrosion is a more harmful one, and it is
difficult to predict the occurrence of a pitting corrosion or to prevent it. Pitting failures may lead to
sudden fractures of metal components and catastrophic accidents. Therefore, research on pitting
corrosion behavior of aluminum alloys has attracted much attention. Tian-hong Zhang
[1] studied the
behavior of pitting corrosion of hard aluminum alloy LY12CZ, it is believed that the nitrate solution
can accelerate the repair of the oxide film to improve the corrosion resistance of the material. Zhu [2]
pointed out that the acidic environment in corrosion pits caused the metal on the inner wall of
corrosion pits to remain in an active state, so that the anodic dissolution continued, which accelerated
the enlargement of the size of corrosion pits and the deepening of pit depth. Jiang [3] studied the
galvanic corrosion caused by the contact between aluminum alloy and titanium alloy, analyzed the
effect of corrosion on mechanical properties and fracture mechanism of the aluminum alloy. Yang
[4]
studied the corrosion characteristics of aluminum alloy 5083 in seawater through experiments and
found that when the cathode potential is negative to -1.15V, hydrogen evolution occurs and the
324
Li, Z., Lv, S., Liu, Z. and Zhang, W.
Effect of Surface Quality on Pitting Corrosion Behavior of Aluminum Alloy 2A12.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 324-329
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

aluminum alloy dissolves. Li et al.
[5] found that the relationship between mass loss due to corrosion
and corrosion time shows a law of power function. Jun-guang He et al. [6] compared the pitting
behavior of pure aluminum and aluminum alloys and found that the alloy can improve the corrosion
morphology. Liu et al. [7] pointed out that Cl‾ is the active factor that induces pitting corrosion of
aluminum alloys, and the polar cathodic protection potential range of 5083 aluminum alloys in
seawater is obtained by polarization experiments. Li [8] used the scanning vibration electrode
technique (SVET) to measure in situ the tiny area of pitting corrosion of 5083 aluminum alloy in
seawater to obtain the variation of surface potential gradient. Mao-fei Zhang [9] performed
experimental research and numerical analysis of aluminum alloy pitting corrosion based on near-field
dynamics.
Many factors will affect the pitting corrosion behavior of aluminum alloys, including the
composition of the alloy, the corrosive medium, the pH of the medium and the ambient temperature.
Parts of the aircraft undergo various types of processing before assembly, such as pressure
processing, metal cutting, EDM, thermal processing and so on. Different processing results in
different surface quality of parts. Surface roughness is one of the important characteristics of surface
quality. There are many microscopic peaks and valleys on the surface of a part, and surface
roughness characterizes this microscopic geometric error. When a part is in contact with some kind
of specific corrosive medium, the corrosive liquid may remain in the valley, and continuous
corrosion will occur, causing localized corrosion and gradual pitting. If the pits continue to develop
in the depth direction below the surface of the structure, the continuity of the material will be
destroyed. This will weaken the strength of the part and cause premature failure. Pits may also be the
origin of stress corrosion crack. Therefore, the influence of the surface quality of parts on the
occurrence and development of pitting corrosion has become a topic of great research value. Walter
et al [10] studied the effect of surface roughness on metastable pitting of magnesium alloy during
initiation stage. Li et al. [11] studied the effect of surface roughness on corrosion behavior of pure
copper in 3.5%NaCl solution. It was found that there was a positive correlation between the
roughness of specimens and the corrosion velocity. Wang [12] used electrochemical methods to
study the effect of surface roughness on the early pitting behavior of 304 stainless steel. Hou and Sun
[13] studied the effect of surface roughness on corrosion resistance and resistivity of high-purity
silver tape. Zhang and Wang et al [14] studied the effect of surface state on corrosion and stress
corrosion behavior of alloy 690TT.
In this paper, we present and discuss the results obtained from accelerated pitting corrosion test
done on aluminum alloy 2A12 in order to grasp the effect of surface quality on the occurrence and
development of pitting corrosion.
2. Experimental procedures
In a laboratory environment, accelerated corrosion experiments of aluminum alloy 2A12 were carried
out with surface roughness of the test specimen as a control variable. The incubator is used to control
the corrosive ambient temperature and the treated test specimen was immersed in a formulated
quantitative corrosion solution. Controlling different corrosion durations, the effect of surface quality
on the occurrence and development of pitting corrosion of the test specimen under different ambient
temperatures and different corrosion durations was observed by using the method of potentiodynamic
scanning.
2.1. Materials
The high-strength aluminum alloy 2A12 was used as the material for test specimens. The chemical
composition is shown in Table 1.
Effect of Surface Quality on Pitting Corrosion Behavior of Aluminum Alloy 2A12
325

Table 1. Chemical composition of aluminium alloy 2A12.
Element Si Fe Cu Mn Mg Ni Zn Ti Aluminium
Percentage ≤0.5 ≤0.5 3.80~
4.90
0.30~
0.90
1.20~
1.80
≤0.1 ≤0.3 ≤0.15 Balance
2.2. Test specimens
The test specimen was cut by wire cutting, its shape designed as a dog bone, as shown in Figure 1.
After cutting, the original specimens were processed: the aluminum-clad layer and the oxide layer
were removed, cleaned and dried; the specimens were divided into two groups A and B. The group A
was polished with 500# water sandpaper, and the group B is gradually polished with 500#, 1000#,
1500# water sandpaper. Scan the area of the specimen to be corroded using a Shapix 3D scanner. The
results show that the maximum difference between the peak and valley values of surface roughness
of group A is 8.9 μ m, while that of group B is 4 μ m.
Figure 1. Shape and size of the test specimen. Figure 2. Corrosion morphology.
2.3. Experimental corrosion medium
The EXCO (exfoliation corrosion) solution is formulated according to ASTM standards. The
composition is shown in Table 2. The pH of the solution is about 0.4.
Table 2. Composition and ratio of EXCO solution.
Composition
NaCl KNO3 HNO3 (70%)
Content
234g/L 64.9g/L 6.3ml/L
2.4. Experimental equipment
Electrochemical tests were performed using a CS 150 electrochemical workstation. The working
electrode was aluminum alloy 2A12, the reference electrode was composed of a saturated calomel
electrode (SCE) and a salt bridge, and the auxiliary electrode was a Pt electrode. The relative open
circuit potentials were set to -0.2 V and 0.2 V for the initial and end of the scan respectively, the scan
speed was 0.5 mV/s, and the sampling frequency was 5 Hz.
3. Results and discussion
3.1. Corrosion morphology analysis
The upper part of Figure 2 respectively presented the surface morphologies of test specimens from
group A, which underwent corrosion in a 25°C ambient temperature, while the corrosion time was 2
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
326

hours, 4 hours and 6 hours. The lower part, respectively presented the surface morphologies of test
specimens from group B which went the same corrosion environment and corrosion time. As can be
seen from the figure, the corrosion of A and B test specimens is increasing with time. However,
under the same conditions, the degree of corrosion in group A was more serious than that in group B.
This shows that greater roughness causes severe corrosion of the surface. When the other conditions
are kept unchanged, and the ambient temperature is set to 40° C and 55° C, the above experiment is
repeated and the same trend is obtained. Figure 3 respectively shows the surface corrosion state of
Group A and Group B specimens observed under a microscope at an corrosion time of 2hour. As can
be seen from the figure, in the same size area, group A had a number of pits of varying sizes and
were independent of each other, while group B had only one pit, showing that group A corrosion was
more severe than group B.
3.2. Potentiometric scan test
Table 3 shows the self-corrosion potential, self-corrosion current density, and corrosion velocity of
aluminum alloys 2A12 with 2 different surface roughness. According to Table 3, at 25°C, as the
surface roughness of the test specimen decreases, the corrosion potential moves positively from -
0.77786 V in group A to -0.73949V in group B,. The corrosion current density decreased from
0.002082 A/cm
2
in group A to 0.002003 cm
2
in group B, indicating that the ion concentration in the
solution decreased, the conductivity decreased, and the corrosion velocity gradually decreased. This
shows that the surface quality has a direct effect on the pitting corrosion of aluminum alloy 2A12,
and the larger the surface roughness is, the easier the pitting corrosion is. In the process of aluminum
alloy corrosion, because of the sensitivity of aluminum to the corrosive medium with chloride ions,
the chloride ions will replace the hydroxides in the aluminum hydroxide sediments by “replacement”
to form highly soluble corrosion product AlCl
3
and falls off from the surface of the substrate.
Corrosion products cannot accumulate for a long time on the surface of the substrate. With the
extension of time, the pits gradually increase, and corrosion solutions accumulate in the pits, which
constitutes a localized micro-electrochemical corrosion environment, which causes the metal in the
pits to continuously undergo anode dissolution. The pits develop simultaneously in both depth and
radial directions. Contact area between the corrosion solution and the metal become larger, and the
corrosion velocity increases. As the corrosion pit expands in the radial direction, adjacent pits will be
connected to each other to form larger pits, destroying the surface integrity of the material. A large
number of pits develop along the depth direction, making the material within a certain depth a porous
material and destroying the continuity of the material. This will inevitably cause a decrease in the
mechanical properties of the material, resulting in a shortened service life and even an abrupt failure.
The above experiment was repeated while maintaining the other conditions unchanged, and the
ambient temperature was set to 40° C. and 55° C. The same change rule of the electrochemical
parameters was obtained.
From Figure 4, it can be seen that the corrosion velocity is increasing with time. However, the
corrosion velocity in group A increased almost linearly, while in group B, the corrosion velocity
hardly changed within the first 4 hours of the onset of corrosion, but it increased linearly after 4
hours. The reason is that the test specimens of group B have small roughness values and have smooth
surfaces and are not prone to corrosion. Therefore, within 4 hours after the corrosion occurs, the
material-medium contact surface caused by the corrosion changes very little and contributes little to
the increase of the corrosion velocity. With the prolongation of time, when the corrosion develops to
a certain extent, pits on the surface of the test specimen increase, the original microroughness of the
material gradually disappears, the effect on the corrosion velocity tend to disappear, and the material-
medium contact surface begins to rapidly increase. As a result, the corrosion velocity rapidly
increases. Therefore, the difference between the corrosion velocity of Groups A and B also continues
to decrease.
Effect of Surface Quality on Pitting Corrosion Behavior of Aluminum Alloy 2A12
327
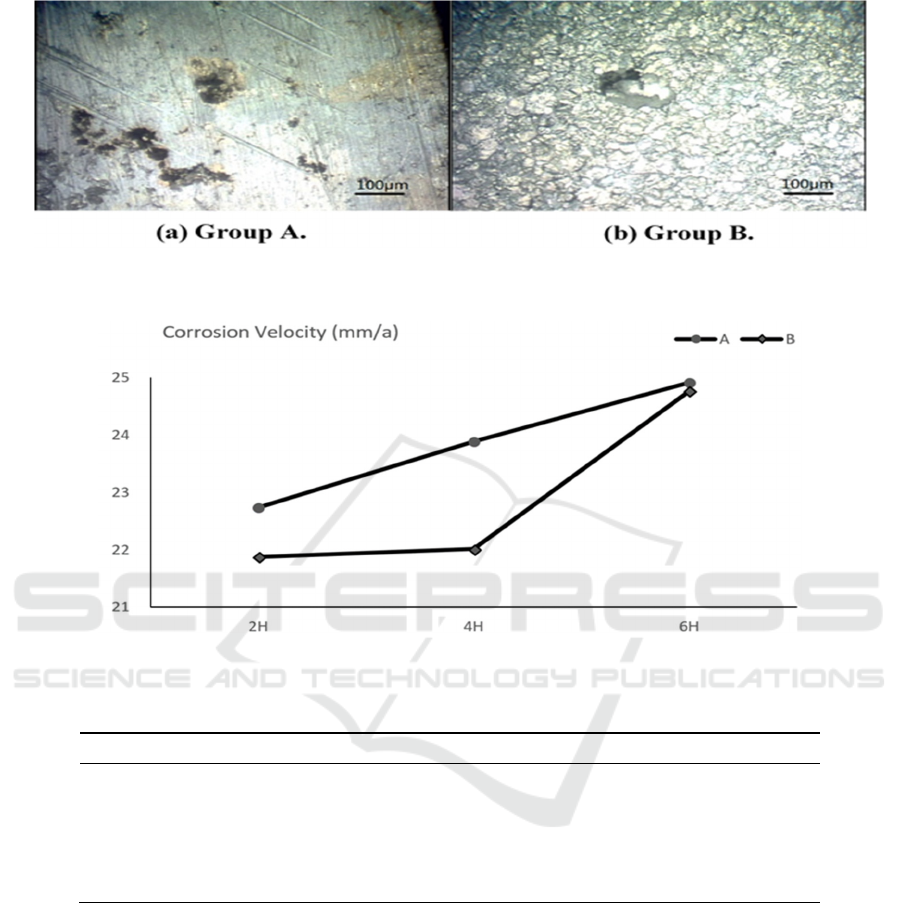
Figure 3.Microscopic corrosion morphology.
Figure 4. Corrosion velocity changes with time.
Table 3. Electrochemical parameters of different surface quality tests specimens in EXCO solution.
Duration(t/ h) Group Icorr (A/cm^2)
Ecorr(V) V(mm/a)
2 A 0.002082 -0.77786 22.736
2 B 0.002003 -0.73949 21.875
4
4
6
6
A
B
A
B
0.002187
0.00202
0.002281
0.002268
-0.74468
-0.73881
-0.75811
-0.72967
23.89
22.01
24.917
24.768
4. Conclusions
The accelerated pitting corrosion test was performed on 2A12 aluminum alloy test specimens to
observe the microscopic surface topography after corrosion, and the effect of the surface quality of
the specimens on the occurrence and development of pitting corrosion was analyzed.
1. The surface quality has a significant effect on the occurrence of pitting corrosion. The corrosion
potential on the surface of the specimens with a large surface roughness value is more negative,
corrosion is more likely to occur, and the corrosion velocity is higher.
2. As corrosion time increases, the corrosion velocity increases continuously.
3. In the initial stage of corrosion, the surface quality has a great influence on pitting corrosion.
With the extension of time, this effect gradually disappears.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
328

Acknowledgment
This work was financially supported by Program 41402020401 and Special Civil Aircraft Program
MJ2016f07.
References
[1] Zhang T H 2004 Effect of Nitrate on Pitting Corrosion of Aluminum Alloy J. Equipment
Environmental Engineering 03: 32-35
[2] Zhu R X 2004 Discussion on Pitting Corrosion of Aluminum Alloy Parts of Aircraft in Coastal
Areas J. Equipment Environmental Engineering 03: 36-39
[3] Jiang S 2014 Research on Galvanic Corrosion of Aluminum Alloy and Titanium Alloy [D].
Xi'an Technological University
[4] Yang T J, Li G M, Chen S, et al 2010 Study of Hull Aluminum Alloy Pitting and Its
Protection Potential J. Equipment Environmental Engineering 7(2) 88-91
[5] Li L, Chen C X, Yang F, et al. 2013 Corrosion Behavior of 0359 Aluminum Alloy in Marine
Atmosphere J. Hot Working Technology 42(2) 28-31
[6] He J G, Wen J B, Sun L M, et al. 2015 Characterization of Pitting Behavior of Pure Al and
Al-7Zn-0.1Sn-0.015Ga Alloy by Cyclic Polarization Technique J. Corrosion Science and
Protection Technology 27(5) 449-453
[7] Liu Z J, Wang J, Zhang P H, et al. 2015 Corrosion Behavior of 5083 Al-alloy in Seawater and
Its Cathodic Protection J. Journal of Chinese Society for Corrosion and Protection 35(3)
239-244
[8] He B, Li L C, et al. 2016 Electrochemical Characteristics in Micro Area of ZL102 Aluminum
Alloy in 3% NaCl Solution J. Equipment Environmental Engineering 13(5) 8-14
[9] Zhang M F 2017 Pitting Corrosion and Its Numerical Simulation Based on Peridynamics [D].
Northwestern Polytechnical University
[10] Walter R and Bobby M 2011 Influence of surface roughness on the corrosion behaviour of
magnesium alloy J. Materials and Design 32:2350-2354
[11] Li W and Li D Y 2006 Influence of surface morphology on corrosion and electronic behavior
J. Acta Mater 54 445-452
[12] Wang M F, Li G D and Du N 2012 Effect of Surface Roughness on Initial Pitting Corrosion
Behavior of 304 Stainless Steel J. Failure Analysis and Prevention 7(2) 86-90
[13] Hou J T, Shun H W, et al. Effect of Surface Roughness on Corrosion Resistance and
Resistivity of High Purity Silver J Precious Metals 201 38(S1) 60-62
[14] Zhang Z M, Wang J Q, et al. 2011 Effects of Surface Condition on Corrosion and Stress
Corrosion Cracking of Alloy 690TT J. Journal of Chinese Society for Corrosion and
protection 31(6) 441-445
Effect of Surface Quality on Pitting Corrosion Behavior of Aluminum Alloy 2A12
329
