
Increased Thermal Conductivity of Mg-1Mn-2Zn-1Nd Alloy
with Aging Time
Y L Zhou
1,*
, J Liu
1
, D M Luo
2
and D C Chen
3
1
School of Mechatronics Engineering, Foshan University, China
2
School of Transportation and Civil Engineering, Foshan University, China
3
School of Materials Science and Energy Engineering, Foshan University, China
Corresponding author and e-mail:Y L Zhou, ylzhou@fosu.edu
Abstract. The thermal conductivity of the Mg-1Mn-2Zn-1Nd alloy aged at 200C for 12, 24
and 48 h was investigated for the applications of heat dissipation. The microstructures were
examined by X-ray diffraction analysis, optical light and scanning electron microscopy. The
thermal conductivity of the Mg alloy was measured at room temperature by laser flash
method. The hardness was measured by with a load force of 29.4 N and dwell time of 30
s.The experimental results indicate that the hardness of Mg-1Mn-2Zn-1Nd alloy first
increases and then decreases with the aging time. The thermal conductivity of the Mg alloy
slowly increases with aging time and its maximum value exceeds the critical value (120
W/(m·k)) of wrought Mg alloys for the applicationsof heat dissipation.The aged Mg-1Mn-
2Zn-1Nd alloy is expected to be a good candidate of heat dissipating alloys.
1. Introduction
The electronic devices have been developed in the direction of high performance, miniaturization and
light weight, and thus higher requirement is needed for the heat dissipation performance of metal fins.
To realize this goal, the metal fins should possess high thermal conductivity. Silver, gold, pure
copper and aluminium (Al) have the best thermal conductivity among the metallic materials [1],
however silver and gold are precious metals with very high price, and copper also has its own
disadvantages: high cost, large weight and poor corrosion resistance. So the currently-used most heat
sinks are made from the light Al alloys. Recently, magnesium (Mg) alloys have attracted increasing
attention and many Mg alloys have been developed as potential thermal materials [2-13] because Mg
has better thermal conductivity (156 W/(m·K)) which is only lower than that of pure Al (237
W/(m·K)) among the commercially-used metallic materials [1] and Mg has relatively lower density
and higher specific heat conductivity. However, the as-cast Mg alloys usually exhibit both poor
thermal and mechanical properties or better thermal property but poor strength [2-4], and the wrought
Mg alloys usually have higher strength but lower thermal conductivity [12, 13], which prevents their
extensive applications of heat dissipation because the heat dissipation materials used for 3C products,
shell of automobile engines and LED radiators demand both higher mechanical and thermal
properties [14]. It seems that the Mg alloys have difficulty inthe applicationsof heat dissipation.
Fortunately, the recent studies have indicated that the aging treatment can improve thermal
performanceof Mg alloys [14-18] and therefore the aged Mg alloys are expected to have a good
Zhou, Y., Liu, J., Luo, D. and Chen, D.
Increased Thermal Conductivity of Mg-1Mn-2Zn-1Nd Alloy with Aging Time.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 215-220
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
215

combination of both mechanical and thermal performance. Previous studies showed that the cast Mg-
Zn-Mn alloy exhibits good heat performance of 125 W/(mK) [10] and the extruded Mg-1Mn-2Zn-
1Nd alloy exhibited the best strength among the Mg-1Mn-2Zn-xNd alloys [19]. Thus the Mg-1Mn-
2Zn-1Nd alloy (mass%) is expected to offer a good combination of both strength and thermal
performance, and the influence of aging treatment on the thermal property of wrought Mg-1Mn-2Zn-
1Nd alloy was studied in this study to check its potential applications of heat dissipation.
2. Experimental
The Mg-1Mn-2Zn-1Nd alloy (mass%) was melted using pure Mg (99.99%), Zinc (99.99 mass%),
Mg-10Mn (99.98 mass%), and Mg-25Nd (99.97 mass%) mother alloys. Pure metals were first put
into a graphite crucible, and the mother alloys were then added at 780C. After melting at
750780C for 0.5 h, the melting cast was finished with a steel mould at 730C. The heat treatment
of homogenization for the cast ingot was performed at 400C for 24 h. The cylinder with the
diameter of 46 mm was hot-extruded to 12 mm at 350C. The chemical compositions of the Mg
alloy were respectively 1.23 mass% of Mn, 2.31 mass% of Zn, 0.81 mass% of Nd and the balance of
Mg, which were checked by X-ray fluorescence spectrometric method. The samples, which were cut
from the hot-extruded bar at cross section, were aged at 200C for 12, 24 and 48 h with water cooling.
The Vickers hardness of the studied alloy was measured by a load force of 29.4 N for 30 s, and five
hardness tests were made for each specimen. The microstructure of the Mg alloy was examined by
light optical microscopy (LM), scanning electron microscopy (SEM) operated at 20 kV and X-ray
diffraction analysis (XRD) using a copper K radiation in the range 2 = 15 85 with 40 kV and 40
mA at the scanning speed of 1/min after the samples were ground with SiC emery papers of up to
3000 grit and polished with 0.5 m diamond powder. The etching solution was composed of 5 ml
nitric acid and 100 ml distilled water.
The specimens with 10 3 mm were machined from the aged alloy bar and the thermal
diffusivity was gauged at room temperature by laser flash method. The averaged density of the
samples measured by Archimedes method was 1.826 g/cm
3
, which was higher than the calculated
density (1.814 g/cm
3
).The Neumann–Kopp Rule was employed to determine the specific heat
capacities of the designed alloy according to the Refs.[20,21]. The thermal conductivity was obtained
using the following equation [2]:
p
C
(1)
Where is the thermal diffusivity (m
2
/s), ρ is the density (g/cm
3
) and C
p
is the specific heat
capacity (J/(gK)) under constant pressure. The experimental results were the averages based on at
least 3 samples.
3. Results and discussion
3.1. Microstructural characterization
Figure 1. presents the XRD results of the Mg-1Mn-2Zn-1Nd alloy aged at 200C for 12, 24 and 48 h,
respectively. It is noticed that both the extruded and aged samples exhibit both phase and Mg
7
Zn
3
,
which indicates that no new precipitate appears during the aging treatment. However, the obvious
texture of extruded sample disappears after aging treatment because the strongest peaks of the aged
alloy are (101) that is the same to that of powder Mg (JCPCS card 35-0821). More peaks of Mg
7
Zn
3
have been detected after the aging treatment, which is possibly due to the increased amount of
Mg
7
Zn
3
duringthe aging treatment.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
216
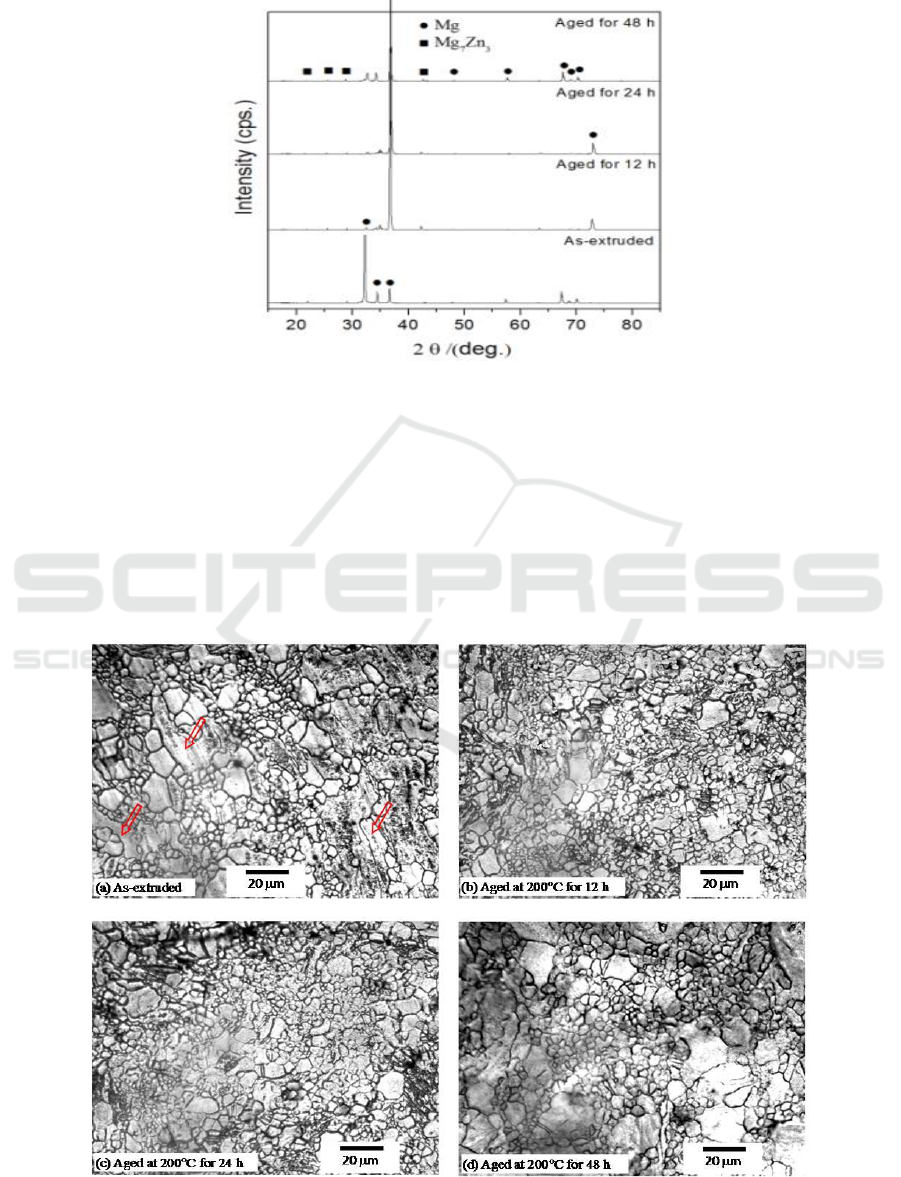
Figure 1. XRD patterns of the Mg alloy.
Figure 2. presents the microstructures of transverse sections of the Mg alloy. It can be noticed that
both the extruded and aged Mg alloy exhibits very fine microstructure. Some unrecrystallized
structure indicated by the arrows exists in the as-extruded sample (Figure 2(a)) while the aged
samples are composed of the similar equiaxed microstructure, which suggests that the re-
crystallization has fully completed after the aging treatment. The Mg
7
Zn
3
is not observed because of
limited resolution of LM and the SEM microstructure proves that Mg
7
Zn
3
forms near the grain
boundaries of the wroughtMg-1Mn-2Zn-1Nd alloy (Figure 3.). The grain sizes of aged alloy slightly
grow with the aging time, especially for the aging time of 48 h.
Figure 2. Microstructures of transverse sections of the Mg alloy.
Increased Thermal Conductivity of Mg-1Mn-2Zn-1Nd Alloy with Aging Time
217
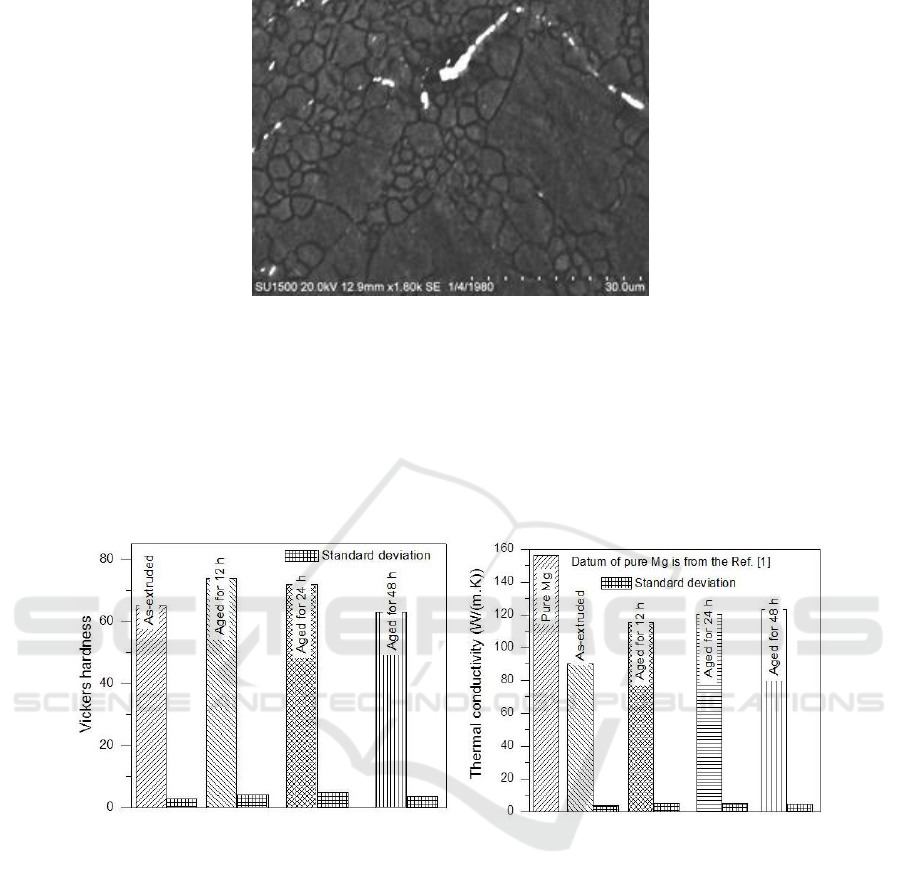
Figure 3. SEM microstructure of the extruded Mg alloy.
3.2. Vickers hardness of the Mg alloy with aging time
Figure 4. depicts the average Vickers hardness of the Mg-1Mn-2Zn-1Nd alloy aged 200C for 12, 24
and 48 h, which first increases and then decreases with the aging time. The slight increase of
hardness for the aged alloy is associated with the formation of new grains from the unrecrystallized
structure and increased amount of Mg
7
Zn
3
during the aging treatment, and the slow reduction of
hardness with the aging time is due to the growth of grain sizes (Figure 2.).
Figure 4. Vickers hardness of the Mg alloy Figure 5. Thermal conductivity of the Mg alloy.
3.3. Thermal conductivity of the Mg alloy with aging time
Figure 5. shows that the thermal conductivity of the Mg-1Mn-2Zn-1Nd alloy aged at 200C for 12,
24 and 48 h, which was calculated from the thermal diffusivity data using Eq.(1), slowly increases
with the aging time and it exceeds 120 W/(mK) when it is aged at 200C for 24 and 48 h. This
change trend is in agreement with the previous studies [15-18]. The thermal conductivity of the
alloys is composed of electronic thermal conductivity and lattice thermal conductivity where the
electrons and phonons are the main heat carrier of the alloys [7]. The lattice defects including
vacancies, dislocations and crystal boundaries, are also the scattering centres of phonons and
electrons that stop the free flow of electrons and accordingly reduce the thermal conductivity of the
alloys [12, 14]. The coarser crystalline size leads to better thermal performance of the Mg alloys [11,
12]. The influence of extrusion texture on the thermal conductivity of ZM51 was investigated [10]
and the texture including dislocation decreases the thermal conductivity of the alloy [10, 12, 13].
Combining with the above microstructures, it is considered that the disappearance of void,
dislocation, texture and growth of grain sizes of aged alloy during the aging treatment are mainly
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
218

responsible for the enhanced thermal conductivity of the alloy with the aging treatment as shown in
Figure 5.
Usually, the ageing treatment leads to the reduction of solute atoms in the Mg matrix and the
subsequent formation of element particles or intermetallic compounds, which would definitely affect
the thermal conductivity of the alloys. However, no new precipitate except Mg
7
Zn
3
and Mg has
been detected by XRD analysis, which is associated with the limited concentration of alloying
elements, Mn, Zn and Nd. Therefore,the changes of defects and grain boundaries of the Mg alloy
during the aging treatment are mainly responsible for the slight increase of thermal conductivity with
the aging time. If new phase precipitates from the Mg alloy, the variation of thermal conductivity
would be abrupt.
According to Figures 4 and 5, it can be observed that the Mg alloy aged at 200C for 24 h has
good combination of both mechanical and thermal properties. The thermal performance of heat
dissipation materials is an essential thermophysical property. The higher thermal conductivity of
metal fins leads to better cooling effect [22], which can prevent the electric (al) equipment from
overheating and prolong the service life. Huawei Technology Co., Ltd., a globe leading manufacturer
of information and technology, demanded that the cast and wrought Mg alloys at least should possess
the thermal conductivity of 100 and 120 W/(mK), respectively[23]. Therefore the Mg-1Mn-2Zn-1Nd
alloy aged at 200C for 24 and 48 h meets this requirement forthe wrought Mg alloys and is expected
to be a good candidate of heat dissipating alloys.
4. Conclusions
The thermal conductivity of the Mg-1Mn-2Zn-1Nd alloy aged at 200C for the different aging time
was studied at room temperature by laser flash method. The experimental results indicate that the
thermal conductivity of the Mg-1Mn-2Zn-1Nd alloy slowly increases with the aging time and
exceeds the required critical value (120 W/(mK)) of wrought Mg alloys, thus the aged Mg-1Mn-
2Zn-1Nd alloy is expected to be a good candidate for the application of heat dissipation.
Acknowledgment
The work was financially supported by the Science and Technology Innovation Platform of Foshan
City, Guangdong Province, China (Grant No. 2014AG10009 and 2016AG100341). And the special
gratitude was expressed to Professor Lei Wang from Northeastern University, China for his kind help
during the hot-extrusion process.
References
[1] Hu F and Chen Z 2009 Calorimetric technology and determination of thermal properties
(Hefei: China University of Science and Technology Press China)p239241
[2] Yamasaki M and Kawamura Y 2009 Scripta Mater.60 264
[3] Rudajevová A, Staněk M and Lukáč P 2003 Mater. Sci. Eng. A341 152
[4] Chen C J, Wang Q D and Yin D D 2009 J. Alloys Compd.487560
[5] Li Y, Xiao Z, Li Z, Zhou Z, Yang Z and Lei Q 2017 J. Alloys Compd. 7231162
[6] Wang C, Liu Z, Xiao S and Chen Y 2016 Mater. Sci. Techno. 32(6) 581
[7] Su C, Li D, Ying T, Zhou L, Li L and Zeng X 2016 J. Alloys Compd.685114
[8] Pan H, Pan F and Yang R 2014 J. Mater. Sci.493107
[9] Zhong L, Peng J, Sun S, Wang Y, Lu Y and Pan F 2017 J. Mater. Sci. Techno.3392
[10] Yuan J, Zhang K, Li T, Li X, Li Y, Ma M and Luo P 2012 Mater. Des.40 257
[11] Yuan J, Zhang K, Zhang X, Li X, Li T, Li Y, Ma M and Shi G 2013 J. Alloys Compd.57632
[12] Ying T, Zheng M Y, Li Z T and Qiao X G 2014 J. Alloys Comp.608 19
Increased Thermal Conductivity of Mg-1Mn-2Zn-1Nd Alloy with Aging Time
219

[13] Ying T, Zheng M Y, Li Z T, Qiao X G and Xu S W 2015 J. Alloys Compd.621250
[14] Peng J, Zhong L, Wang Y, Yang J, Lu Y and Pan F 2015 J. Alloys Compd.639 556
[15] H Pan, F Pan, J Peng, J Gou, A Tang, L Wu and H Dong 2013 J. Alloys Compd. 578493
[16] Huang Q, Tang A, Ma S, Pan H, Song B, Gao Z, Rashad M and Pan F 2016 J. Mater. Eng.
Perform. 25(6)2356
[17] Wang C, Cui Z, Liu H, Chen Y, Ding W and Xiao S 2015 Mater. Des.8448
[18] Li B, Hou L, Wu R, Zhang J, Li X and Zhang M 2017 J. Alloys Compd.722772
[19] Zhou Y L, Li Y, Luo D M, Ding Y and Hodgson P 2015Mater. Sci. Eng. C4993
[20] Leitner J, Voňka P, Sedmidubský D and Svoboda P 2010 ThermochimActa497 7
[21] Lindemann A, Schmidt J, Todte M and Zeuner T 2002 Thermochim Acta382269
[22] RudajevováA, Buch F V and Mordike B L 1999 J. Alloys Compd.292 27
[23] Peng J, Zhong L, Wang Y, Lu Y and Pan F 2015 Mater. Des.87914
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
220
