
Synthesis and Characterization of Heteropoly Complex of
Magnesium-substituted Zinc-centred Undecatungstate
Ligand
X S Lv, F W He, S B Cui and Q Y Wu
*
School of Biomedical and Chemical Engineering, Liaoning Institute of Science and
Technology, Benxi 117004, Liaoning, China
Corresponding author and e-mail: Q Y Wu, qywu@lnist.edu.cn
Abstract. New Magnesium-substituted heteropoly undecatungstozincate complex with
Keggin structure, K
8
[Mg (H
2
O) ZnW
11
O
39
] ·13H
2
O, was synthesized by the stepwise
acidification and the stepwise addition of materials. The product was characterized by ICP,
IR spectrum, UV spectrum, X-ray power diffraction and thermal analysis.
1. Introduction
Heteropoly acids and heteropoly complexes, negatively charged early transition metal oxide clusters,
are formed by inorganic metal–oxygen cluster anions with special structures, and they can be applied
in many areas, such as catalysts for organic reactions, dopants in sol-gel matrixes, corrosion resistant
coatings, membranes in selective electrodes, gas detection apparatus, liquid and solid electrolytic
cells, solid-state electrochromic devices, sensors and hydrogen-oxygen fuel cells [1-7]. The synthesis
of mixed heteropoly complexes is a frontal work in the basic studies on heteropoly compounds. 11
tungsten unsaturated heteropoly anion as a ligand can form mixed heteropoly anion with transition
elements, rare earth elements and main group element ion. The heteropoly anion structure is 1:12 the
Keggin structure of lost as a W-O
d
. Due to the heteropoly complexes have the catalytic activities and
antiviral properties with the reactions of some organic synthesis [8-15]. The synthesis of novel
complex is still attracting people's attention. The synthesis and characterization of the new heteropoly
complex K
8
[Mg (H
2
O) ZnW
11
O
39
] ·13H
2
O are described in the paper.
2. Experimental
2.1. Synthesis
K
8
[Mg (H
2
O) ZnW
11
O
39
] ·13H
2
O was synthesized according to recent literature source [16]. 0.11
mol sodium tungstate (Na
2
WO
4
·2H
2
O) was dissolved in 200 mL distilled water. The solution was
adjusted to pH~6.3 with acetic acid and was heated to boil. After that, 40 mL hot weak acidic
aqueous solution which contained 0.01 mol zinc sulfate solution (ZnSO
4
·7H
2
O) was added dropwise
to the above solution with stirring. The mixture was continuously heated at 100°C for 30 min, a
solution of 30 mL 0.01 mol of magnesium sulfate (MgSO
4
) was dropwise added to the mixture with
stirring. The pH was readjusted to 5.0 and stirring was continued for 1.5 h. Finally, the cooled
202
Lv, X., He, F., Cui, S. and Wu, Q.
Synthesis and Characterization of Heteropoly Complex of Magnesium-substituted Zinc-centred Undecatungstate Ligand.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 202-206
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
solution was extracted with 30mL absolute alcohol. A white oily matter was obtained. Dissolving it
with boiled distilled water, and then adding 25g KCl and stirring for several minutes at room
temperature. The oily product was precipitated, and then extracted by dissolving-cooling method for
several times.
2.2. Instruments and reagents
Infrared spectrum (IR) was recorded on a Perkin- Elmer 1730 FT/IR spectrometer with KBr pellets.
The UV spectrum was measured on a PERSEE TU-1901 spectrophotometer in water solution. X-Ray
powder diffraction analysis was obtained on a BRUKER D8 ADVANCE X-ray diffractometer. The
thermal stability of the sample was investigated using simultaneous thermogravimetry (TG) and
differential thermal analysis (DTA) techniques. The measurement was performed using a NETZSCH
STA 449C thermal analyzer in a nitrogen stream, with a scanning rate of 10°C min
-1
. An 8410 ICP
spectrometer was also used.
2.3. Elemental Analysis
Potassium, magnesium, zinc, and tungsten were by ICP spectrometry. The water content was
determined by thermogravimetry. Elemental anal.(%) calcd. for K
8
[Mg(H
2
O)ZnW
11
O
39
]·13H
2
O: K
9.86, Mg 0.73, Zn 1.98, W 61.28, H
2
O 7.6; Found: K 9.77, Mg 0.72, Zn 1.95, W 60.88, H
2
O 7.55.
3. Results and discussion
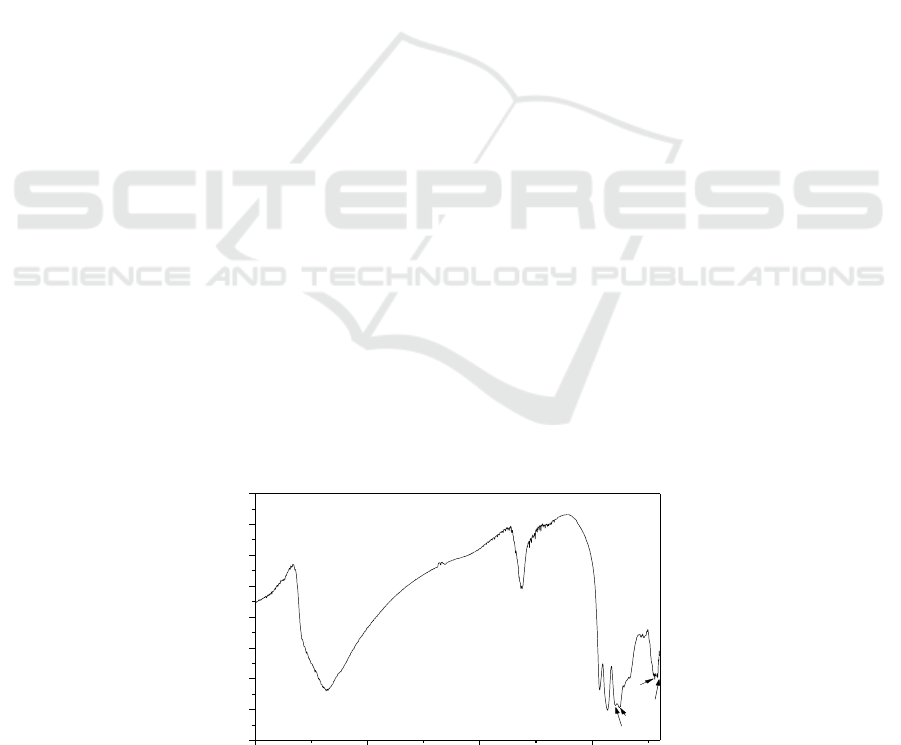
3.1. IR Spectra
Infrared spectroscopy is an effective method for studying the structure [17]. The best feature area of
the spectrum is around 1100–700 cm
-1
are observed, due to the absorption of metal-oxygen
stretching vibrations [18-19]. In the IR spectrum of K
8
[Mg(H
2
O)ZnW
11
O
39
]·13H
2
O as shown Figure
1, there are four bonds: ν
as
(W-O
d
), 933 cm
-1
; ν
as
(W-O
b
-W) , 862 cm
-1
; ν
as
(W-O
c
-W) , 797 and 748 cm
-
1
; ν
a
(Zn-O
a
) , 443 ans 418 cm
-1
. These characteristic vibration patterns of K
8
[Mg (H
2
O)
ZnW
11
O
39
] ·13H
2
O indicate the heteropoly complex still maintains Keggin structure.
The vibrational frequencies fall in the sequence of ν
as
(W-O
d
) >ν
as
(W-O
b
-W)> ν
as
(W-O
c
-W),
which are assigned to W-O
d
stretching, stretching of W-O
b
-W inter bridges between corner-sharing
WO
6
octahedra and bending of
W-O
c
-W inter bridges between edge-sharing WO
6
octahedra at
700~1100 cm
-1
. These bands can be easily identified and confirm the formation of this hybrid
molecular complexes Additionally, two evident peaks in the range of 3351 and 1623 cm
−1
correspond to the stretching vibration of O-H bonds and the bending vibration of H-O-H bonds,
respectively.
4000 3000 2000 1000
30
40
50
60
70
80
90
100
110
400
Transmittance
(%)
Wavenumber(cm
-1
)
3351
1623
933
862
794
748
443
418
Figure 1.IR spectrum of K
8
[Mg (H
2
O) ZnW
11
O
39
]·13H
2
O.
Synthesis and Characterization of Heteropoly Complex of Magnesium-substituted Zinc-centred Undecatungstate Ligand
203
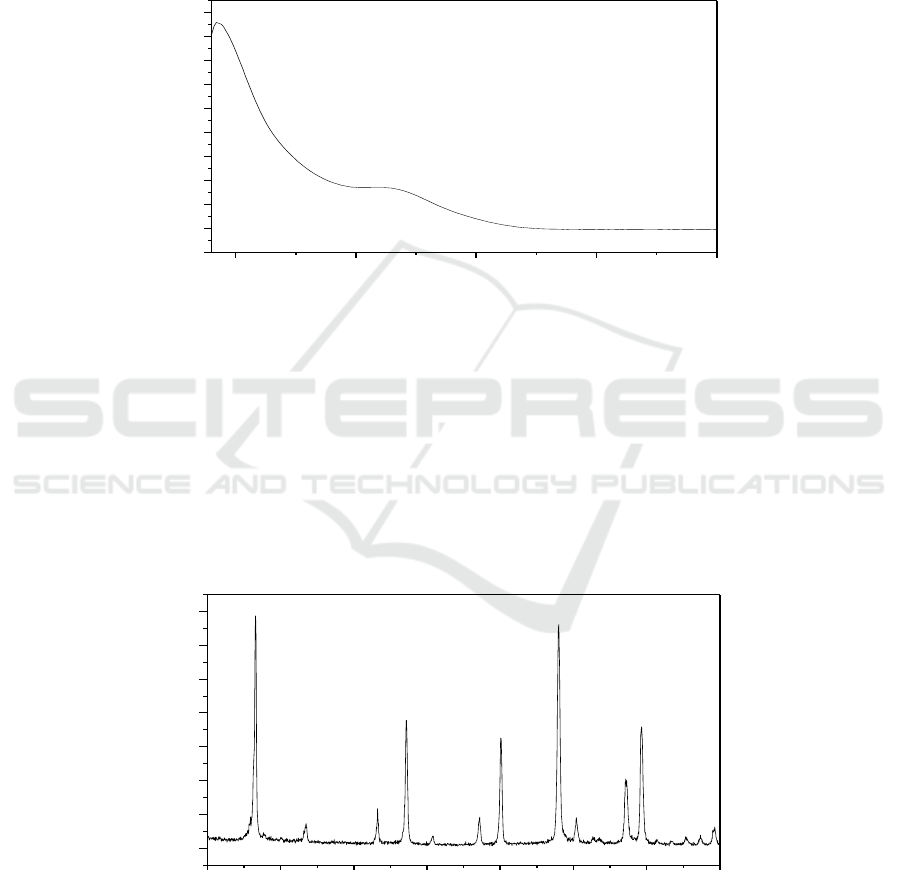
3.2. UV Spectra
The heteropoly complexes are generally characterized by oxygen-to-metal (O-M) charge transfer
bands, which appear in the UV region below 400 nm. The UV spectrum data for the complex is given
in Figure 2. There is an intense absorption peak at 192 nm, which can be considered as the terminal
oxygen (O
d
→W). Similarly, a relatively weak absorption peak at 261 nm can be regarded as the
charge-transfer of the bridge oxygen to metal atoms (O
b
/O
c
→W). So there is evidence of the
characteristic bands of Keggin structure of the heteropolytungstates [20].
200 250 300 350 400
-0.2
0.0
0.2
0.4
0.6
0.8
1.0
1.2
1.4
1.6
1.8
192
Absorbance
Wavelength(nm)
261
Figure 2.UV spectrum of K
8
[Mg(H
2
O)ZnW
11
O
39
]·13H
2
O.
3.3. X-ray powder diffraction
X-ray diffraction analysis is used extensively to study the structure of heteropoly complexes [21-22].
The result of X-ray powder diffraction (XRD) of the product is shown in Figure 3. The most intense
peak exists at about 8.28° for K
8
[Mg (H
2
O) ZnW
11
O
39
] ·13H
2
O. The Bragg reflection peaks which
exist in four ranges of 2θ (i.e. 7~10°, 16~22°, 25~30° and 33~38°) are the characteristic peaks of the
heteropoly complex with Keggin structure, showing that the target product has high crystallinity.
5 10 15 20 25 30 35 40
0
2000
4000
6000
8000
10000
12000
14000
intensity
2θ(dgree)
Figure 3. XRD pattern of K
8
[Mg (H
2
O) ZnW
11
O
39
]·13H
2
O.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
204
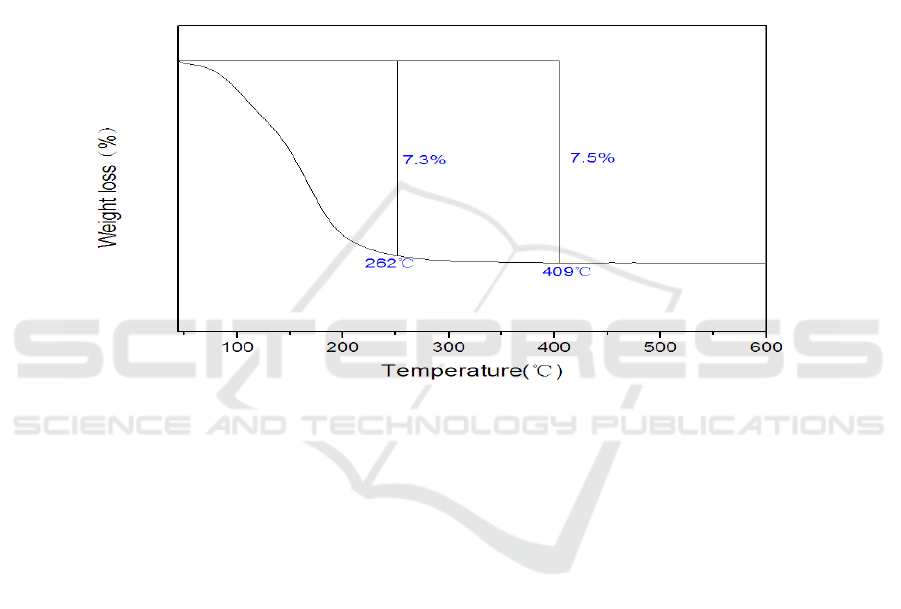
3.4. Thermal analysis
Figure 4 shows the TG curve of the heteropoly complex. Heteropoly complex is usually obtained
with a large amount of water of crystallization [23]. Three types of water molecule can be
distinguished in these solids: hydration water, zeolite water and structural water. The TG curve
shows a major weight loss of 7.5% for the heteropoly complex below 409°C , which demonstrates
that 14 water molecules have been lost for the heteropoly complex. There are almost 13 molecules of
hydration water below 262°C for the heteropoly complex and finally the loss of 1 molecule of
structural water at 409°C for the heteropoly complex. So the accurate molecular formula of the
heteropoly complex
can be assigned as K
8
[Mg (H
2
O) ZnW
11
O
39
]∙13H
2
O.
In general, we take the temperature of the exothermic peak of DTA curve as the sign of their
thermostability. In the curve, there is an exothermic peak at 470°C , at which the product decomposes.
Figure 4. TG curve of K
8
[Mg (H
2
O) ZnW
11
O
39
] ·13H
2
O.
4. Conclusions
The synthesis and characterization of the heteropoly complex of magnesium with
undecatungstozincate is reported in this paper. The composition and structure of the complex was
determined by means of ICP and XRD. TG curve show that the weight loss of the product is a two-
step process. The results show that the general formula is K
8
[Mg (H
2
O) ZnW
11
O
39
] ·1 3H
2
O and the
structure of the product derives from the Keggin structure. The IR and UV spectra of the complex
were investigated. And the synthesized product possess potential application prospect for catalysis.
Acknowledgements
This work was supported by the Liaoning Provincial Natural Science Foundation of China
(201602404) and Scientific Research Foundation of Liaoning Institute of Science and Technology
(RXYJ2015001).
References
[1] Busche C, Vilà-Nadal L, Yan J, Miras H N, Long D L, Georgiev V P, Asenov A, Pedersen R
H, Gadegaard N, Mirza M M, Paul D J, Poblet J M and Cronin L 2014 Nature 515 545
[2] Yin Q S, Tan J M, Besson C, Geletii Y V, Musaev D G, Kuznetsov A E, Luo Z, Hardcastle K I
and Hill C L 2010 Science 328 342
[3] Wu Y L, Shi R F, Wu Y L, Holcroft J M, Liu Z C, Frasconi M, Wasielewski M R, Li H and
Stoddart J F 2015 J. Am. Chem. Soc. 137 4111
[4] Xie Z R, Wu H, Wu Q Y and Ai L M 2018 RSC Adv. 8 13984
Synthesis and Characterization of Heteropoly Complex of Magnesium-substituted Zinc-centred Undecatungstate Ligand
205

[5] He P L, Xu B, Wang P P, Liu H L and Wang X 2014 Adv. Matter. 26 4339
[6] Li L L, Han H Y, Wang Y H, Tan H Q, Zang H Y and Li Y G 2015 Dalton Trans. 44 11429
[7] Shi D Y, He C, Qi B, Chen C, Niu J Y and Duan C Y 2015 Chem. Sci. 6 1035
[8] Tong X, Wu X F, Wu Q Y, Zhu W M, Cao F T and Yan W F 2012 Dalton Trans. 41 9893.
[9] Huang T P, Tian N Q, Wu Q Y and Yan W F 2015 Soft Matter. 11 4481
[10] Kourasi M, Wills R G A, Sha A A and Walsh F C 2014 Electrochim. Acta 127 454
[11] Miao J, Liu Y W, Tang Q, He D F, Yang G C, Shi Z, Liu S X. and Wu Q Y 2014 Dalton
Trans. 43 14749
[12] Wu X F, Huang T P, Wu Q Y and Xu L 2016 The Royal Society of Chemistry 45 271
[13] Tong X, Tian N Q, Wu W, Zhu W M, Wu Q Y, Cao F H, Yan W F 2013 J. Phys. Chem. C
117 3258.
[14] Tong X, Zhu W M, Wu Q Y, Qian X Y, Liu Z, Yan W F and Gong J 2011 J. Alloys Compd.
509 7768.
[15] Tong X, Tian N T, Zhu W M, Wu Q Y, Qian X Y, Cao F H., Yan W F and Gong J 2012 J.
Alloys Compd. 544 37
[16] Wu Q Y, Zhai Y C 1996 J. Rare Metals. 15 257
[17] Wu X F, Li Y Y, Wu Q Y, Ding H and Yan W F 2014 Phys. Chem. Chem. Phys. 16 24598
[18] Qian X Y, Tong X, Wu Q Y, He Z Q, Cao F H and Yan W F 2012 Dalton Trans. 41 9897
[19] Miao H, Xu X, Jun W W, Wan H X, Zhang Y, Zhu D R and Xu Y 2014 Inorg Chem. 53
2757
[20] Huang T P, Wu X F, Wu Q Y and Cao F H, Funct 2015 Mater. Lett. 8 1550041.
[21] Liu Y W, Yang X, Miao J, Tang Q, Liu S M, Shi Z and Liu S X 2014 Chem. Commun. 50
10023
[22] Fide R G, Rapko B, Saxton R J and Domaille P J 1986 J. Am. Chem. Soc. 108 2947
[23] Wu X F, Wu W, Wu Q Y and Yan W F 2017 Langmuir 33 4242
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
206
