
Investigation of Thermal Decomposition Kinetic of
Polyethylene 100 Compounds with Kissinger Model
G J Huang
*
, S P Li, W W Ye, B Yang, M D Li and M L Xin
Guangzhou special pressure equipment inspection and research institue, Guangzhou
510663, China
Corresponding author and e-mail: G J Huang, huangguojia@163.com
Abstract. The thermal stability and non-isothermal degradation kinetics of the polyethylene
100(PE100) compounds were studied by thermogravimetric(TG) and derivative
thermogravimetric(DTG) analyses using multiple heating rates (2, 5, 10, 20 °C/min) under
nitrogen gas atmosphere. The thermal features of PE100 compounds was examined and the
average activation energy(Ea) thus obtained with Kissinger method is 333.79 kJ/mol.
1. Introduction
With the development of modern energy, the use of natural gas to replace coal-fired fuel in
production and life is becoming more and more widespread, and the scale and number of gas pipeline
networks are also rapidly increasing[1-2]. As an important part of the distribution system, the
performance of the natural transmission pipeline is related to the safety of the entire system. Among
the medium and low-pressure gas transmission and distribution pipelines in cities and towns,
polyethylene (PE) has become a preferred material as gas pipe because of its good welding
performance, chemical resistance, toughness and weather resistance, and good resistance to rapid
crack propagation[3-4]. The development and application of polyethylene pipes have undergone four
stages. In the 1940s, in order to replace the traditional metal pipe, people produced the first
generation polyethylene pipe material PE63 with corrosion resistance, but its environmental stress
crack resistance was poor. In the 1960s, as the relative molecular mass and the content of comonomer
were increased, the second-generation polyethylene pipe PE80 with long-term service life was
developed with slow crack resistance. However, it has been found that the relative molecular mass is
too high to reduce the processing performance of the material, while the increase in the comonomer
content leads to a decrease in the rigidity of the material. In order to take into account both
processing and use properties, the PE100, a third generation PE pipe material, was developed. The
important performance of gas-fired polyethylene pipes is long-term service life. In the past decade or
more, one of the developments in PE pipe materials has been to lock in with slow crack growth or
stress cracking resistance in search of new applications. Based on the third-generation PE100, the
fourth-generation PE pipe material, PE100RC, was further developed through molecular structure
design to improve the resistance to slow cracking. The representative product is a bimodal molecular
weight distribution of hexene copolymerized PE100-RC.
Huang, G., Li, S., Ye, W., Yang, B., Li, M. and Xin, M.
Investigation of Thermal Decomposition Kinetic of Polyethylene 100 Compounds with Kissinger Model.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 183-188
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
183

Polyethylene pipeline contains a flammable special medium such as natural gas, and several gas
fire accidents have occurred in life [5-6]. Once a fire breaks, polyethylene will burn and decompose
at high temperatures, and the remaining pipes will also become garbage. How to understand the
thermal decomposition mechanism of polyethylene pipe materials is of great significance to the
safety protection of pipes and the reuse of waste polyethylene. Its efficient reutilization has a growing
importance these years due to the increased demand for resource recycling and environmental
protection. In general, plastic waste has been mainly disposed of by landfill or incineration, but these
processes are not fully acceptable under current international policy, which focuses on efficient
recovery of raw material and energy. Pyrolysis and gasification processes are promising routes for
optimal upgrading from waste. Moreover, pyrolysis of plastic, based on the decomposition of
polymers at different temperatures, allows the treatment of polymers with simultaneous
decomposition and separation [7-8].
Thermal degradation of polymers has great interest as an alternative source of energy or chemical
raw materials, as well as it contributes to the solution of environmental problems [9]. The
determination of the parameters of the thermal decomposition process by means of TG techniques
allows the development of the recycling process of these materials in an industrial scale. Thermal
behavior of plastics can be improved by knowing thermal degradation kinetic [10-11]. Pyrolysis of
plastic can transform it into valuable chemicals [12], and it becomes a popular way to dispose waste
plastics. Meanwhile, thermal degradation is usually occurring during the polymer processing.
Designing and implementation of the pyrolysis or process for polymers depends primarily on kinetic
analysis [13]. Precised kinetics heavily relies on the reliable kinetic triplets, activation energy (Eα),
preexponential factor (A) and reaction model (f (α)) [14]. A thermogravimetric analysis technique is
an excellent way for studying the kinetics of thermal degradation. TG provides the pathway to
determine the macroscopic kinetics of these processes such as the information on activation energy
and kinetic model [15].
In this work, the thermogravimetric study of polyethylene compounds was realized using
non-isothermal method in order to determine the apparent activation energy with Kissinger model.
2. Measurement and method
2.1. Measurement
The PE100 compound (Borstar® HE 3490LS) was purchase from Borouge company. PE100 samples (~10
mg) were subjected to TG analysis in an inert atmosphere of nitrogen. NETZSCH 209 F1 TG
analyzer was used to measure and record the sample mass change with temperature over the course
of the decomposition reaction. TG curves were obtained at four different heating rates (2, 5, 10,
20 °C /min) from room temperature 700 °C.
2.2. Kinetic analysis method
Kinetic analysis of a thermal decomposition process is usually expected to produce a satisfactory
kinetic description in terms of the reaction model and the Arrhenius parameters. Typically, the
generalized form of kinetic equation can be expressed as Eq.1
(1)
Where α is the degree of conversion and it ranges from 0 to 1. Where k(T) and f(α) are
temperature dependent rate constant and mass dependent reaction model. The rate constant k(T) is
assumed to follow the Arrhenius law such as k(T)=Aexp(-E
a
/RT), where the temperature and time
coordinates are related as T= T
0
+βt. Eq.1 can be written as following
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
184
(2)
T where A is the pre-exponential factor (1/min-) and Ea is the apparent activation energy (kJ/mol),
respectively, R is the gas constant (8.314 J/mol/K). The differential isoconversional method
suggested by Kissinger [16] is based on Eq. 2 given by the following relation:
(3)
Where T
p
is the peak temperature at the maximum reaction rate. Plot of
versus
should give straight lines, and E
a
is calculated from the slope of the fitted straight line.
3. Results and discussion
3.1. Thermal features of PE100
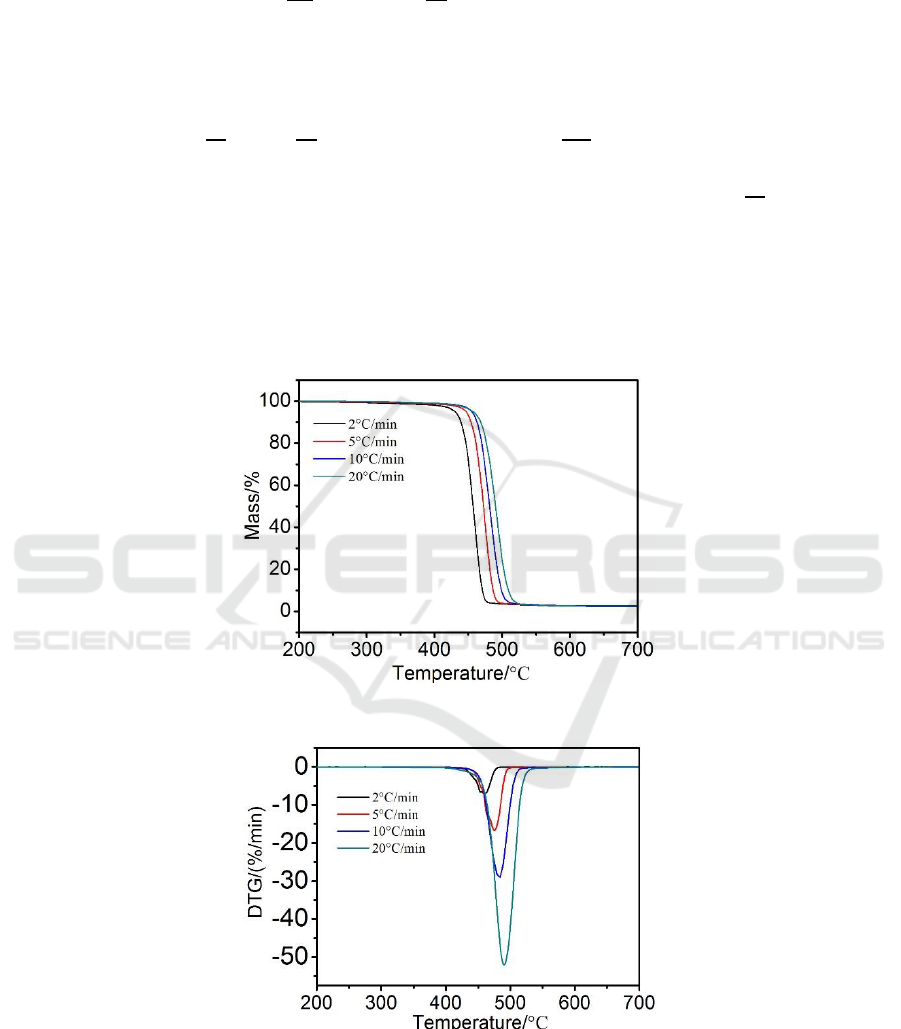
Figure 1.TG profiles of PE100 at four heating rates (2, 5, 10 and 20°C/min).
Figure 2.DTG profiles of PE100 at four heating rates (2, 5, 10 and 20°C/min).
The TG and DTG curves of PE100 at a heating rate of 2, 5, 10 and 20 °C/min in N2 are shown in
Figure 1 and Figure 2 respectively. From TG curves it can be observed that the shape of the weight
loss curve does not change with the variation of heating rate. The TG characteristic temperatures of
PE100 at the different heating rates are illustrated in Table 1. Tp and the decomposition temperature
Investigation of Thermal Decomposition Kinetic of Polyethylene 100 Compounds with Kissinger Model
185
at 5% weight loss (T
5%
) shift to higher temperature with increasing from 2 to 20 °C/min is mainly
due to the time and temperature history subjected to the materials. At higher heating rates, the time
required to reach the decomposition temperature becomes shorter, causing the temperature difference
between the sample inside and outside turning to be larger as well, and subsequently causes thermal
lagging which may delay the sample inside thermal decomposition. It can be seen from Figure 2 that
the DTG peak becomes stronger and wider as heating rate increases from 2 to 20 °C/min, and in the
meantime Tp is promoted from 460 to 490 °C.
Table 1.Characteristic temperatures of thermal degradation of the PE100 compound (HE 3490LS).
T
5%
: the decomposition temperature at 5% weight loss.
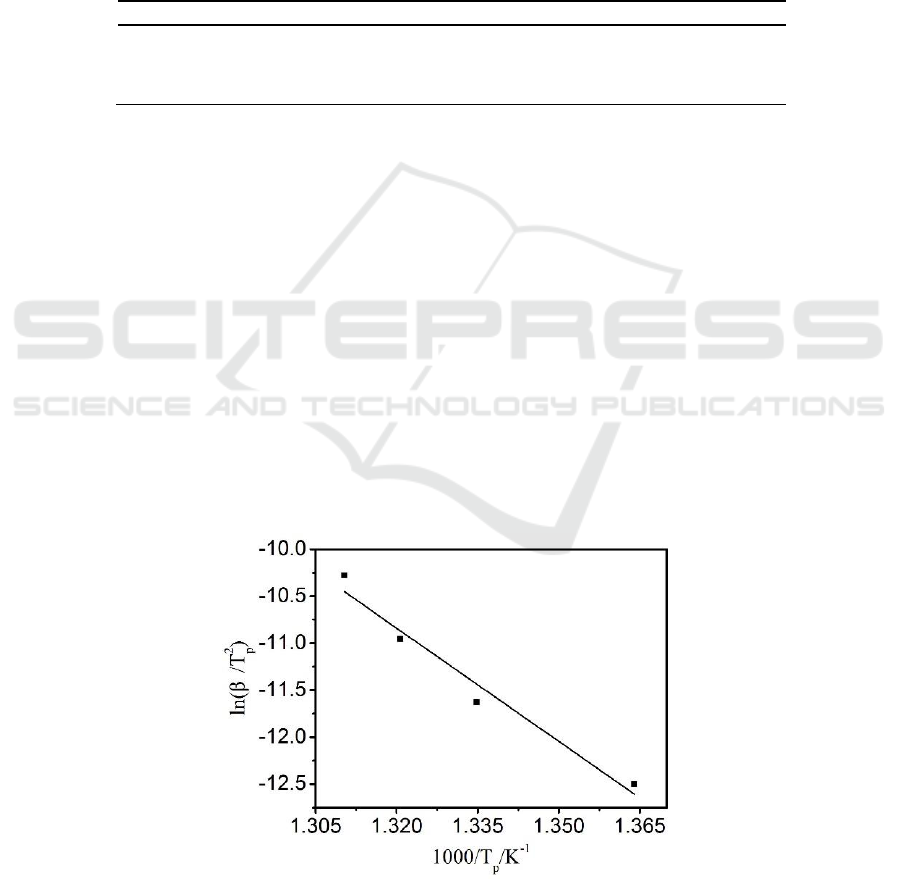
3.2. Kinetic analysis with Kissinger method
This method derives from the correlation between the peak temperature (T
p
) and . Suited for more
than four thermal analytical curves of the derivation type, it is an efficient model-free differential
method. According to Eq.3, the linear fitted plots between
versus
obtained for
PE100 are shown in Figure 3. The results show that the activation energy(Ea) is 333.79 kJ/mol.
Westerhout has examined the pyrolysis kinetics of low-density polyethylene below 450 °C, and also
made a comparison of activation energy(Ea) with literature models and data[17]. The thermal
decomposition Ea for low-density polyethylene varied from 201 to 330 kJ/mol,all of which is lower
than that of PE100 compound with Kissinger method. The above results may be due to the higher
molecular weight. As proper selection of the conversion function is extremely important to formulate
a self-consistent global pyrolysis kinetic model that performs uniformly well in a practically
realizable range heating rates occurring in polymer combustion[18], Kissinger method gives an
average Ea overall the decomposition process.
Figure 3.Kissinger method plots of
versus
.
(°C/ min)
2
5
10
20
T
5%
T
P
241
460
290
476
329
484
336
490
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
186

4. Conclusions
In this work, thermal decomposition kinetics of the PE100 compounds has been investigated, and the
measurements were carried out under N
2
atmosphere at different heating rates. TG and DTG results
show that the thermal features of PE100 compounds are strongly depend on the heating rates with a
single decomposition step. With the Kissinger method, the apparent activation energy (Ea) of PE100
compounds during thermal degradation is 333.79 kJ/mol.
Acknowledgement
The authors would like to thank the Science and Technology Research Programs of Guangzhou City
(Grant No. 201607010258); the Scientific and Technological Project of Guangzhou Quality and
Technical Supervision Bureau (Grant No. 2017KJ21); the Scientific and Technological Project of
Guangdong Provincial Quality and Technical Supervision Bureau (Grant Nos. 2016CT14,
2017CT29).
References
[1] Wei L and Geng P 2016 A review on natural gas/diesel dual fuel combustion,emissions and
performance [J]. Fuel Processing Technology 142 264-278
[2] Hao H, Liu Z, Zhao F, et al. 2016 Natural gas as vehicle fuel in China: A review [J].
Renewable and Sustainable Energy Reviews 62 521-533
[3] Almeida J C, Macieski G C, Zanette L A, Almeida J C, Macieski G C, & Zanette L A, 2015
General guidelines for developing executive design of natural gas urban pipeline networks
and using hdpe valve [J]. Brazilian Journal of Petroleum & Gas 9(1) 19-26
[4] Hua Y, Wu Z J, Xiong Z M,et al. 2014 The developing trends of polyethylene (PE) gas piping
systems[J]. Total Corrosion Control 4 34-40
[5] Brandt A R, Heath G A, Kort E A, et al. 2014 Methane leaks from North American natural gas
systems [J]. Science 343(6172) 733-735
[6] McKain K, Down A, Raciti S M, et al. 2015 Methane emissions from natural gas infrastructure
and use in the urban region of Boston, Massachusetts[J]. Proceedings of the National
Academy of Sciences , 112(7) 1941-1946
[7] Marongiu A, Faravelli T, Bozzano G, Dente M and Ranzi E 2003 Thermal degradation of
poly(vinyl chloride) Journal of Analytical and Applied Pyrolysis 70 519-53
[8] Kiran N, Ekinci E and Snape CE 2000 Recyling of plastic wastes via pyrolysis Resoure
Conservation Recycle 29 273-83
[9] Yang J, Miranda R and Roy C 2001 Using the DTG curve fitting method to determine the
apparent kinetic parameters of thermal decomposition of polymers Polymer Degradation
Stability 73 455–61
[10] Vyazovkin S and Wight C A, 1998 Isothermal and non-isothermal kinetics of thermally
stimulated reactions of solids International Reviews in Physical Chemistry 17 407-433
[11] Friedman H L, 1964 Kinetics of thermal degradation of char-forming plastic from
thermogravimetry.application to a phenolic plastic [J]. Journal of Polymer Science Part C,
6 183-195
[12] Demirbas A 2004 Pyrolysis of municipal plastic wastes for recovery of gasoline-range
hydrocarbons [J]. Journal of Analytical and Applied Pyrolysis 72(1) 97-102
[13] Das P and Tiwari P 2017 Thermal degradation kinetics of plastics and model selection
[J].Thermochimica Acta 654 191-202
[14] Collazzo G C, Broetto C C, Perondi D, et al. 2017 A detailed non-isothermal kinetic study of
Elephant Grass Pyrolysis from different models[J].Applied Thermal Engineering 110
1200-1211
Investigation of Thermal Decomposition Kinetic of Polyethylene 100 Compounds with Kissinger Model
187

[15] Aboulkas A, Harfi K E 2010 A E Bouadili. Thermal degradation behaviors of polyethylene
and polypropylene Part I: Pyrolysis kinetics and mechanisms [J]. Energy Conversion &
Management 51(7) 1363-1369
[16] Kissinger H E 1957 Reaction Kinetics in Differential Thermal Analysis[J]. Analytical
Chemistry 29(11) 1702-1706
[17] Westerhout R W J, Waanders J, Kuipers J A M et al 1997 Kinetics of the Low-Temperature
Pyrolysis of Polyethene, Polypropene, and Polystyrene Modeling, Experimental
Determination, and Comparison with Literature Models and Data [J]. Industrial &
Engineering Chemistry Research 36(6) 1955-1964
[18] Snegirev A Y, Talalov V A, Stepanov V V et al 2017 Autocatalysis in thermal
decomposition of polymers [J]. Polymer Degradation & Stability 137 151-161
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
188
