
Synthesis of 4-Carbazole -7- Thiophen[1,2,5] Thiadiazolo
[3,4-c]Pyridine
Y Liu, X H Zhang, W J Ding and X X Sun
*
Jiangxi Key Laboratory of Organic Chemistry, Jiangxi Science & Technology
Normal University, Nanchang 330013, China
Corresponding author and e-mail: X X Sun, sunxiaoxia77@126.com
Abstract. A novel A-D-A small organic molecule 4-Carbazole -7- thiophen-
[1,2,5]thiadiazolo[3,4-c]pyridine was synthesized by two step with good yield. The
compound with good electronic and optical properties may be a candidate for D-A-D type
organic light-emitting diodes.
1. Introduction
Designs and synthesis of novel conjugated organic molecules and polymers have drawn widely
attention upon the past two decades, owing to develop new synthetic methodology and commercial
optoelectronic device which can be put into use, take organic field-effect transistors
(OFETs),[1]polymer light-emitting diodes (PLEDs)[2-4]and polymer solar cells (PSCs)[5-6]for
example.
To carry out the application of full-color advanced flat panel displays, the development of
suitable green-, white-, blue- and red-emitting (RGB) organic molecules and polymers[7] will be
important. However, the molecular structure, molecular weight, and purity of the polymer are usually
uncertain, leading to differences in the properties of different batches of materials, which may lead to
batch instability during industrial production in the future. Compared with polymer materials, the
molecular structure and molecular weight of organic small-molecule solar cell materials are well-
defined, and these materials are relatively easy to separate and purify, have high purity, and have
good batch stability in the preparation process. However, the development of organic small molecule
solar cell is relatively slow, the types of materials reported in the literature are scarce, and the
photoelectric conversion efficiency of the battery is also very low.
2,1,3-benzothiadiazole(BT) has been widely used by researchers to construct D-A conjugated
molecules because of its strong coplanarity, strong electron-withdrawing ability, high oxidation-
reduction potential and good stability. And D-A type conjugated molecules are widely used in
organic photoelectric materials.[8] During the past few years organic small molecules and conjugated
polymer materials constructed with BT structural units as acceptor materials have achieved great
achievements in organic solar cells, which promoted the development of organic solar cells to a large
extent. In the area of solution-processable organic small molecules and conjugated macromolecules
constructed by the BT cell, the Li Yongfang Research Group has done massive and systematic
research work and has obtained a series of very significant research results. In 2006, the research
group constructed a bulk heterojunction solar cell of small molecule Figure 1 using thiophene,
122
Liu, Y., Zhang, X., Ding, W. and Sun, X.
Synthesis of 4-Carbazole -7- Thiophen[1,2,5] Thiadiazolo [3,4-C]Pyridine.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 122-126
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
triphenylamine and BT as structural units. The photoelectric conversion efficiency of the compound
was 0.26%. In subsequent work, the research group constructed new molecules S2 and S3 by
adjusting the chemical bond of S1, the photoelectric conversion efficiency reached 1.23% when the
bulk heterojunction solar cell was prepared in which S2 was the donor and [6,6]-phenyl-C71-methyl
butyrate (PC71BM) as a receptor material. In 2011, the research group replaced thiophene in the
molecule with thiophthene which has greater electron-donating capability and coplanarity. The small
molecules S4 to S6 are constructed as shown in the figure. It was found that by changing the
molecular structure, the absorption wavelength of the material can be effectively broadened and the
photoelectric conversion efficiency of this class of compounds can reach 1.44%.
Figure 1. Pyridalthiadiazole-Based Narrow Band Gap Chromophores.
During solution processing, conjugated molecules including pyridal- [2,1,3]thiadiazole (PT)
sections synthesized by Guillermo C. Bazan[9] have recently come up to 6.7% efficiencies of power
conversion, molecular bulk-heterojunction (BHJ) organic photovoltaics. Due to the significance of
the systems which belong to the novel type of molecule and establishing a more effective pathway to
make solid-state performances better, they start to methodically change the structure of the molecule
and assess the property−framework relationships. D1-PT-D2-PT-D1 compounds were synthesized by
stille coupling reaction where donor meaning a relatively rich electronic ratio compared to PT.
Physical properties were measured on a combination of absorption spectroscopy, thermal gravimetric
analysis, cyclic voltammetry, solubility and differential scanning calorimetry. Alter to end-capping
D1 units due to better control the electronic energy levels both in solution and in steric hindrance.
Figure 2. DAA organic molecules.
Synthesis of 4-Carbazole -7- Thiophen[1,2,5] Thiadiazolo [3,4-C]Pyridine
123
Ken-Tsung Wong et al.[10] used dicyanovinylene as an electron withdrawing moiety while 2,1,3-
benzothiadiazole as another acceptor. Three DAA small-molecule organic solar cells were
strategically designed and synthesized through still coupling reaction by changing various electron-
donating moieties. At the level of molecules and single compounds, the photophysical and
electrochemical properties of the compounds and the energy levels of the molecules were
systematically studied. In addition, the connection between the structure of the molecules and their
properties was clearly obtained. The UV maximum absorption bands were near 700-800 nm which
assigned to the ICT transition. The photoelectric power conversion efficiency (PCE) of solar cell
materials was up to 6.6+0.2%, which provides reference data for the design of small molecule
compounds used in photovoltaic cell materials.
2. Experimental section
Without being purified, all chemicals and reagents were received from commercial sources. The
solvents used in the chemical synthesis is purified by distillation. All chemical reactions are carried
out in the atmosphere of argon or nitrogen.
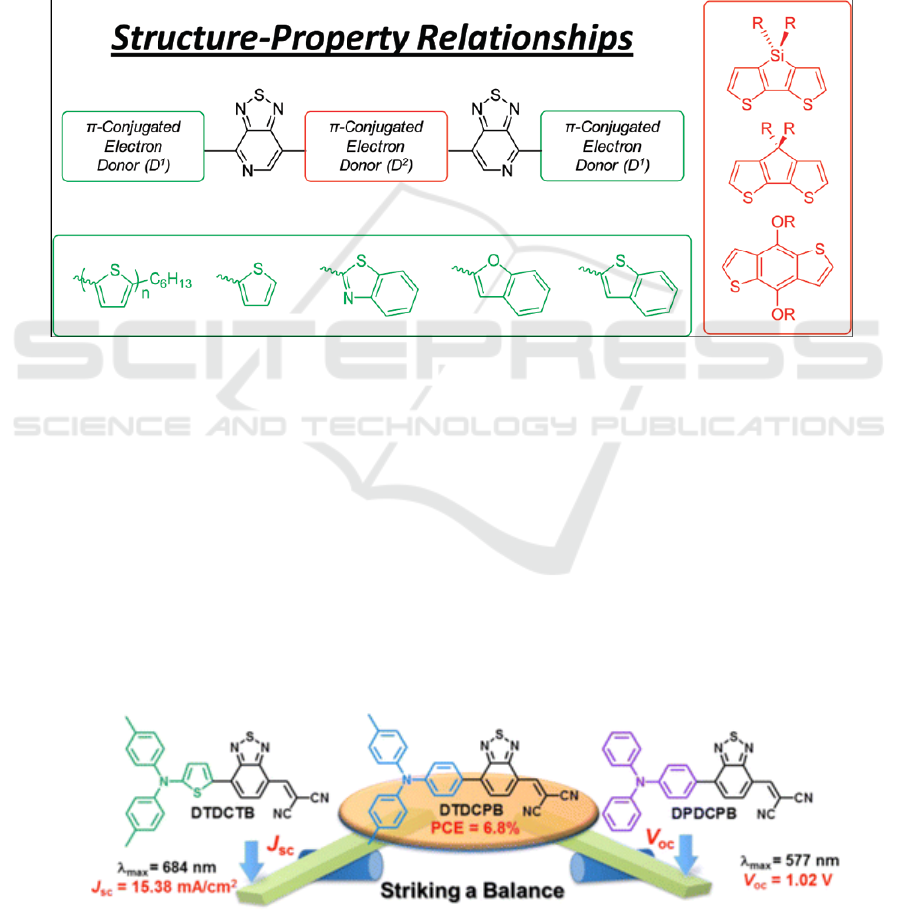
2.1. Synthesis of 7- bromo-4-Carbazole-[1,2,5]thiadiazolo[3,4-c]pyridine
(1)
To a 25.0 ml single-mouth flask was added 2,7-dibromopyridothiadiazole (295.0 mg, 1.0 mmol),
carbazole (200.6 mg, 1.2 mmol) at room temperature. The cuprous iodide (19.0 mg, 0.1 mmol),
sodium carbonate solid (552.0 mg, 4.0 mmol) and 1,10-phenanthroline (36.0 mg, 0.2 mmol), which
had been previously dehydrated and dried in a vacuum drying oven were added to the above flask,
15.0 mL of purified toluene was used as the solvent (deoxidation). The reaction system was refluxed
overnight under argon protection. TLC showed that the starting material point had disappeared and
the reaction was stopped. Then at nature air cooling, Silica gel was directly added to the reaction
system, and the solvent was evaporated on a rotary evaporator to obtain a red-brown solid. Column
separation was performed using petroleum ether and ethyl acetate as the eluent (V (petroleum ether):
V (ethyl acetate) = 15:1) and the second fraction was collected and spin-dried to afford a red solid
125.0 mg in 66.0% yield.
1
H NMR (400 MHz, CDCl
3
, TMS): δ (ppm) 8.72(s, 1H), 8.12-8.10(d, 2H),
7.51-7.59(d, 2H), 7.31-7.41(d, 4H);
13
C NMR (100 MHz, CDCl
3
, TMS): δ 145.34, 133.76, 126.11,
124.45, 123.92, 120.96, 121.16, 115.63, 112.51, 111.57.
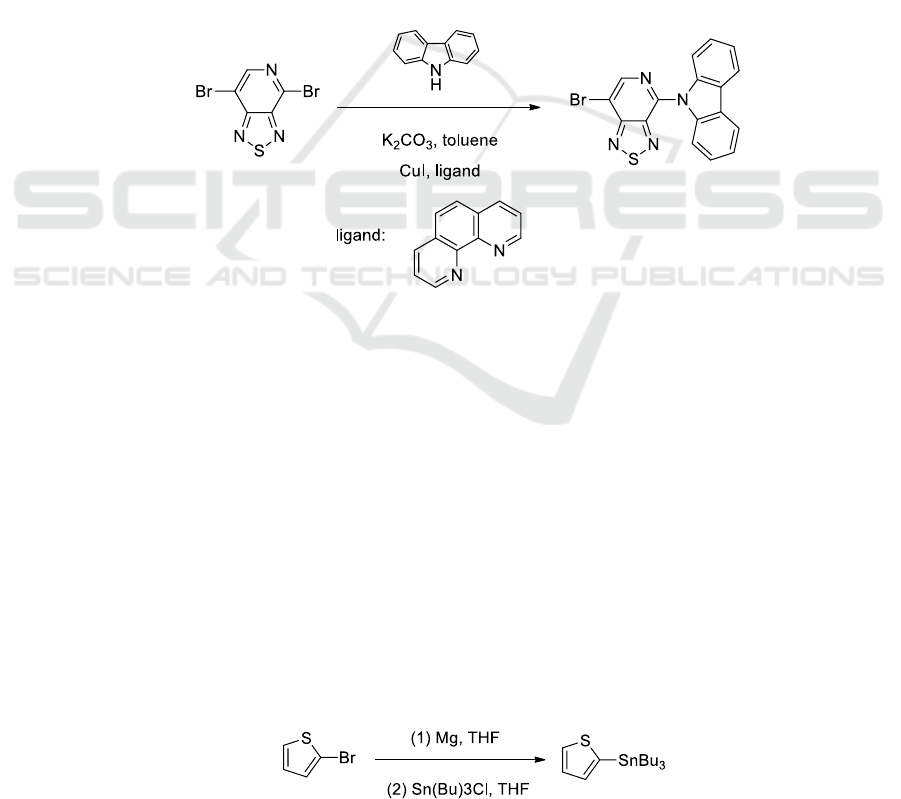
2.2. Synthesis of Tributyl(2-thienyl)stannane
(2)
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
124
In a two neck round bottom flask charged with magnesium (880 mg, 36.8 mmol) in degassed THF
(3.0 mL) was slowly added 2-bromothiophene (5.0 g, 30.67 mmol, 1 equiv) in (30 mL degassed THF)
to reach reflux temperature. Further stirring at reflux temperature was performed for 1 h. The product
was used in the next step without further purification. The solution was transfer to -78°C . After
stirring at the same temperature for 1 h, Tri-n-butyltin chloride (1m in hexane, 10.96 g, 33.74 mmol)
was added to the reaction mixture and stirred at the same temperature for 0.5 h. Then the temperature
was slowly rise to room temperature while stirring was maintained for 12 h. The aqueous solution of
Na
2
CO
3
was added into the mixture and the aqueous phase was then extracted with n-hexane three
times and washed with 50ml brine. The organic layer was dried over anhydrous magnesium sulfate
and filtered. Yellow liquid was obtained after evaporation of the solvent. The residue was purified by
column chromatography through Al
2
O
3
using n-hexane to give 9.7 g product in 85.0% yield as slight
yellow oil.
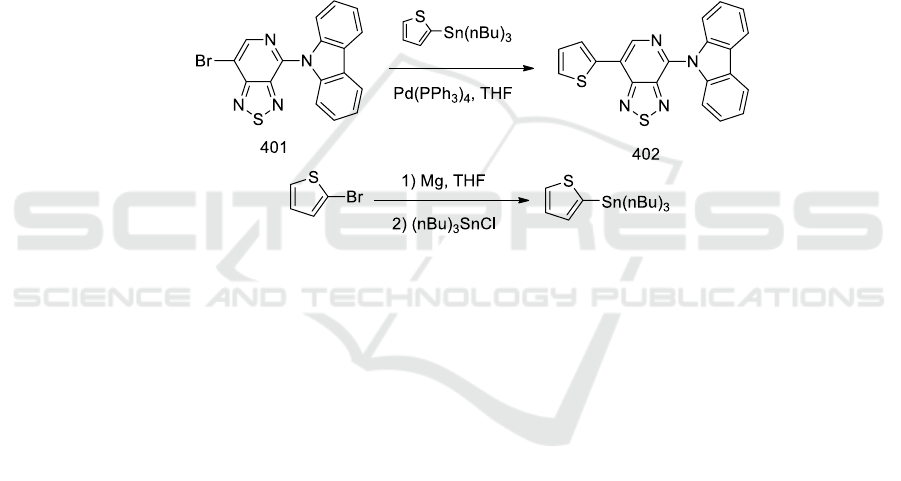
2.3. Synthesis of 4-Carbazole -7- thiophen-[1,2,5]thiadiazolo[3,4-c]pyridine
(3)
To a 10.0 mL single-mouth flask was added 7- bromo-4-Carbazole-[1,2,5]thiadiazolo[3,4-c]pyridine
(40.0 mg, 0.11 mmol) and Tributyl(2-thienyl)stannane (156.0 mg, 0.42 mmol) at room temperature.
To the flask was added 5.0 ml of purified tetrahydrofuran as a solvent and activated under argon
atmosphere at room temperature for 30.0 min. Tetrakistriphenylphosphine palladium (Pd(PPh
3
)
4
)
(20.0 mg, 0.01 mmol) was added to the flask to continue activating for 15 min. Then, the reaction
system was transferred to an oil bath at 80°C and refluxed overnight. The reaction was stopped when
TLC test showed that the starting point disappeared and the new point was generated. After cooling
this system to room temperature, silica gel was added to the reaction, and the solvent was spin-dried
on a rotary evaporator to obtain a bright red solid. Column separation was performed using petroleum
ether and ethyl acetate as the eluent (V (petroleum ether): V (ethyl acetate) = 10:1) and the second
fraction was collected and spin-dried to collect a red solid 28.0 mg in 70.0% yield.
1
H NMR (400
MHz, CDCl
3
, TMS): δ(ppm) 8.91(s, 1H), 8.21-8.33(m, 3H), 7.61-7.63(d, 2H), 7.55-7.56(d, 2H),
7.35-7.42(m, 4H), 7.27-7.29(m, 1H).
13
C NMR (100 MHz, CDCl
3
, TMS): δ 126.11, 124.45, 123.92,
120.96, 121.16.
3. Result and discussion
A novel A-D-A small organic molecule 4-Carbazole -7- thiophen-[1,2,5]thiadiazolo[3,4-c]pyridine
was synthesized by two step with good yield. [1,2,5]thiadiazolo[3,4-c]pyridine was used as an
electron withdrawing moiety while carbazole and thiophene as the two different electric donor
moiety. The UV−vis absorption spectra of the two molecules in THF are drawed in Figure 3. As
shown from Figure 3 that after the incorporation of carbazole on pyridine- thiadiazole, the maximum
UV absorption wavelength is significantly red shifted to 458 nm. After the introduction of a
thiophene fragment, the UV absorption of the molecule will be followed by a red shift of 20 nm to
Synthesis of 4-Carbazole -7- Thiophen[1,2,5] Thiadiazolo [3,4-C]Pyridine
125
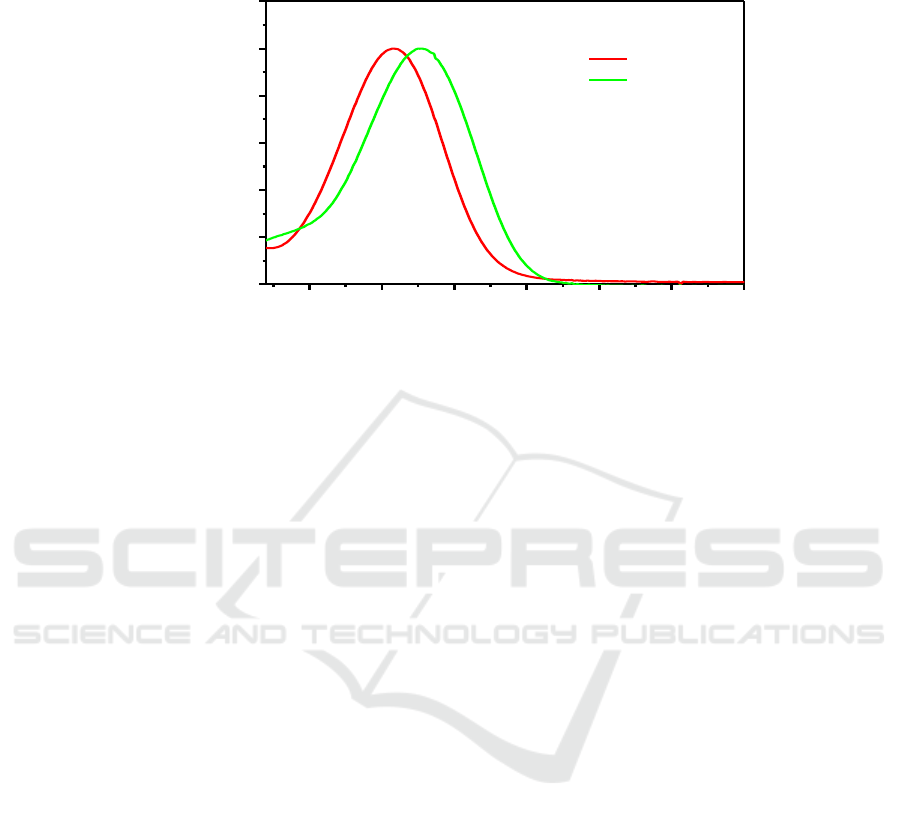
478 nm. If we then introduce an electron-donating segment and introduce the above-mentioned
fragments into small molecules, the molecules should be applicable to many fields.
Figure 3. the UV absorption spectra of small molecules M1 and M2.
4. Conclusions
A novel A-D-A small organic molecule 4-Carbazole -7- thiophen-[1,2,5] thiadiazolo[3,4-c]pyridine
was synthesized with good yield by two steps. [1,2,5]thiadiazolo[3,4-c]pyridine was served as an
electron withdrawing moiety while carbazole and thiophene as the two different electric donor
moiety. In order to boost device efficiencies, further engineering of molecular structures and
morphology optimization are currently underway.
Acknowledgments
The authors are grateful for the financial support of the National Natural Science Foundation of
China (No.21462018), the Science Fund of the Technology Office of Jiangxi, China (2009ZDS11100
and 20132BBE50024), and Jiangxi Science and Normal Technology Normal University Program for
Science Research Innovation Team (2013CXTD002)
References
[1] Li Y 2012 Acc. Chem. Res. 45 723
[2] Mei J, Diao Y, Appleton A L, Fang L and Bao Z 2013 J. Am. Chem. Soc. 135 6724.
[3] Grimsdale A C, Chan K L, Martin R E, Jokisz P G and Holmes A B 2009 Chem. Rev. 109 897.
[4] Yang X, Xu X and Zhou G 2015 J. Mater. Chem. C 3 913.
[5] Facchetti A 2011 Chem. Mater. 23 733.
[6] Chen J and Cao Y 2009 Acc. Chem. Res. 42 1709.
[7] Zhang L J, Hu S J, Chen J W, Chen Z H, Wu H B, Peng J B and Cao Y 2011 Adv. Funct.
Mater. 21 3760.
[8] He C, He Q G, He Y J, Li Y F, Bai F L, Yang C H, Ding Y Q, Wang L X and Ye J P 2006 Sol.
Energy Mater. Sol. Cells. 90 1815.
[9] Zachary B H, Gregory C W, Thomas van der P and Guillermo C B 2012 J. Am. Chem. Soc.
134 3766.
[10] Chen Y H, L Lin Y, Lu C W, Lin F, Huang Z Y, Lin H W, Wang P H, Liu Y H, Wong K T,
Wen J, Dean J M and Seth B D 2012 J. Am. Chem. Soc. 134 13616.
400 450 500 550 600 650 700
0.0
0.2
0.4
0.6
0.8
1.0
1.2
M1
M2
Norm. Absorbance(a.u)
Wavelength(nm)
458nm
478nm
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
126
