
Robust Transparent Super Hydrophobic Coatings and
Control of Interface Structure
J Zhang
1
, H B Xu
1
, Y Huang
1, 2
, Z H Zhou
1, 2, *
and S Shen
3
1
College of Materials, Xiamen University, Xiamen, Fujian, 361005, China
2
Fujian Key Laboratory of Advanced Materials, Xiamen, Fujian, China 361005
3
CSIRO Manufacturing, Clayton, VIC 3168, Australia
Corresponding author and e-mail: Z H Zhou, zzh@xmu.edu.cn
Abstract. Mechanically robust, transparent and superhydrophobic coatings on glass surface
are critical for building and automotive self-cleaning function. In this paper, by using acidic
SiO
2
sol as a binder and mixing two different sizes of hydrophilic SiO
2
nanoparticles with the
binder, mechanically robust transparent coatings with micro/nano hierarchical structure were
prepared. The influence of mixing process on interface structure was studied. Results show
that abrasive resistance of micro/nano hierarchical structural coatings depends on the control
of voids and holes on the interface. Water contact angle (WCA) of the micro/nano
hierarchical structural coatings reaches >150° when modified by fluoroalkylsilane. Under
condition of load of 1 kg/cm
2
, WCA can still maintain >120° after 200 cycles of mechanical
abrasion, showing excellent wear resistance and application prospect.
1. Introduction
Micro/nano hierarchical structure and low surface energy are two critical factors for
superhydrophobic surfaces [1]. In recent years, applications of SiO
2
nanoparticles to prepare
superhydrophobic surfaces have received widespread attention [2-4]. This is mainly because that
aggregation of SiO
2
nanoparticles can provide multi-scale hierarchical structure -- nano-scale
structure constructed by primary particle and micro-scale structure constructed by particle
aggregation [5]. However, abrasive resistance and transparency of micro/nano hierarchical structure
are still key issues [6-9].
Abrasive resistance of micro/nano hierarchical structure constructed by nanoparticle aggregation
without binder is weak due to small contact area between hierarchical structure and substrate [10-11].
To improve abrasive resistance, Cai et al. [12] sprayed commercial hydrophobic SiO
2
nanoparticles
(R974, 12 nm) onto glass substrate pre-coated with organic binder (epoxy resin) to fabricate a
superhydrophobic surface with a WCA of 154.7°; Xu et al. [13] used inorganic SiO
2
sol as binder,
dispersed 2500 nm/400 nm or 400 nm/50 nm dual-sized SiO
2
particles in acidic SiO
2
sol to construct
micro/nano hierarchical structure, and prepared a superhydrophobic coating with a WCA of 160°.
Though the abrasive resistance is improved, but the coating is not transparent because of the big
Zhang, J., Xu, H., Huang, Y., Zhou, Z. and Shen, S.
Robust Transparent Super Hydrophobic Coatings and Control of Interface Structure.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 93-99
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
93

particle size of SiO
2
, and the application is limited.
In this paper, we focused on the influence of mixing process of SiO
2
nanoparticles and binder on
interface structure between micro/nano structure and glass. The voids and holes on the interface were
controlled, and mechanically robust transparent superhydrophobic coatings were successfully
prepared on glass.
2. Experimental
2.1. Preparation of micro/nano structural coatings
Reagents: tetraethoxysilane (TEOS), ethanol, coupling reagent (KH560), deionized water, dilute
nitric acid, commercial hydrophilic SiO
2
nanopowder A380 (7 nm, Degussa) and A200 (25nm,
Degussa), and fluoroalkylsilane (1H, 1H, 2H, 2H-perfluorodecyl three chlorinated silane) were used
as received.
The preparation of acidic SiO
2
sol is based on our previous report [14]. TEOS was hydrolyzed in
aqueous-ethanol solution of dilute nitric acid and KH560 was added as coupling reagent.
0.2 g of A380 SiO
2
nanopowder and 0.2 g of A200 SiO
2
nanopowder were added to 20 g of
ethanol, stirred for 50 min, and sonicated for 1 h to prepare SiO
2
nanopowder dispersion sol (labeled
as AA sol). 2.5 g of AA sol was mixed with 1.0 g of the prepared acidic SiO
2
sol and 4.0 g of
isopropanol, sonicated for 1 h to prepare a mixed sol of SiO
2
nanopowder and acidic SiO
2
sol
(labeled as AAS sol).
A commercial green glass of 50 mm x 50 mm x 3.2 mm pre-treated by polished and
decontaminated was used as substrate. Before coating, glass substrate was chemically activated by
ultraviolet ozone irradiation (UVO irradiation, 10 min) to form a highly active hydroxyl polar surface
[15]. The sol was coated onto glass substrate by spin coating with a KW-4A homogenizer (speed 600
rpm, time 10 s), followed by pre-curing at 80 °C for 20 min and solidification at 180 °C for 1 h.
Monolayer coatings were prepared as follows. In order to study interface structure of monolayer
coatings, (1) acidic SiO
2
sol, (2) AA sol and (3) AAS sol were coated on glass substrate to fabricate
monolayer coatings of (1) SiO
2
gel (labeled as sample S), (2) SiO
2
nanoparticle aggregation (labeled
as sample AA), and mixed SiO
2
nanoparticle with SiO
2
gel (labeled as sample AAS), respectively.
Bilayer coatings were prepared as follows. In order to study interface structure of bilayer coatings,
at first, acidic SiO
2
sol was coated as under-layer. After air-dried and UVO irradiated, (4) AA sol and
(5) AAS sol were coated as upper layer to fabricate bilayer coatings of (4) SiO
2
gel/SiO
2
nanoparticle
aggregation (labeled as sample S/AA), and (5)SiO
2
gel/mixed SiO
2
nanoparticle with SiO
2
gel
(labeled as sample S/AAS), respectively.
Fluoroalkylsilane modification was implemented by chemical vapor deposition according to
reference [16]. The coated glass was placed in the reactor with polytetrafluoroethylene as inner tank,
on the bottom of which was distributed three droplets of 1H, 1H, 2H, 2H-perfluorodecyl three
chlorinated silane. There was no direct contact between the coated glass and the droplets. The reactor
was heat treated at 120 °C for 2 h, after natural cooling, the coated glass was removed and heat
treated at 150 °C for 1.5 h.
2.2. Characterizations
Abrasive resistance was conducted according to ISO 5470-1:2016 using a reciprocating linear
wear-resistance instrument (x-5750-J, Shenzhen Xinhengsen Trading Co., Ltd.), under condition of
load of 1 kg/cm
2
and reciprocating 200 times. Interface structure before and after abrasion was
observed using a scanning electron microscope (SEM, SU-70, Hitachi) with an accelerating voltage
of 5 kV. Since conductivity of glass sample was poor, sample surface was treated with gold spray
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
94
before SEM detection. Atomic force microscopy (AFM, Multimode 8, BRUKER) was used to
characterize 3D topography and roughness of sample surface in tapping mode. An UV/VIS/NIR
spectrometer (Lambda 750 S type, Perkin Elmer) was used to analyze optical transmittance. A
contact angle measurement instrument (JCY-4, Shanghai Fangrui) was used to measure static water
contact angle (water droplet 4 μl).
3. Results and discussion
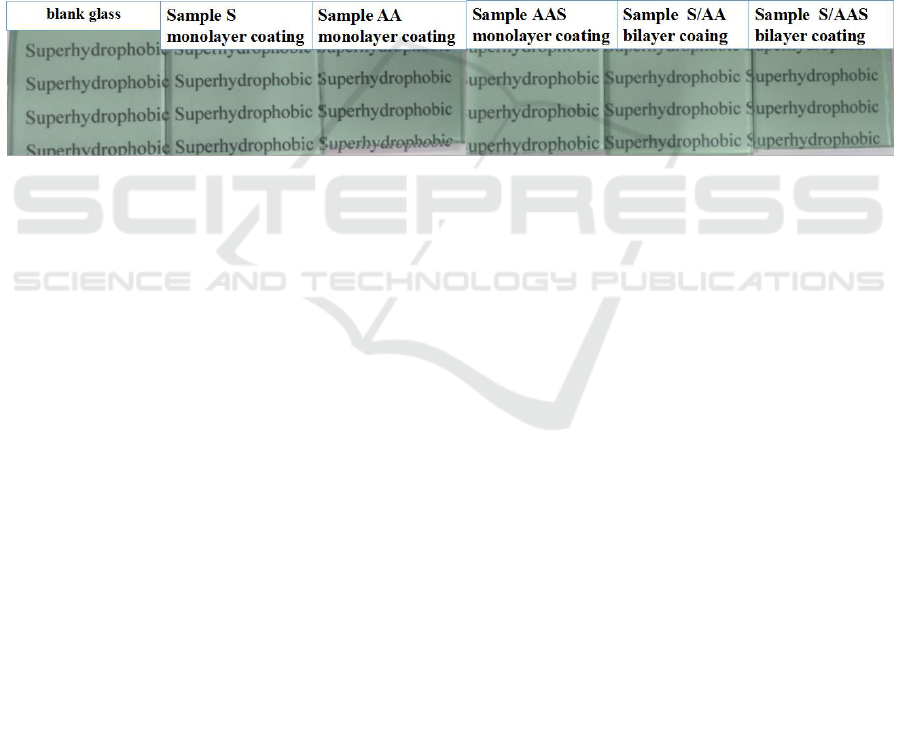
The photos of as-prepared samples are shown in figure 1. The samples with monolayer coatings of
sample S, sample AA and sample AAS are transparent, and the visible light transmittances (TLs) are
75.53%, 75.51% and 74.85%, respectively. The reason of sample AA with a coating constructed by
aggregation of two types of SiO
2
nannoparticles of A380 (7 nm) and A200 (25 nm) being transparent
is mainly because of the small nanosize of SiO
2
particles used. Compared with the superhydrophobic
surface constructed with large nanosize of SiO
2
particles (2500 nm/400 nm or 400 nm/50 nm
dual-sized SiO
2
particles) in Reference 13, the transparency is significantly improved.
Figure 1.Photos of the prepared samples.
The samples with bilayer coatings of sample S/AA and sample S/AAS are also transparent, and
the TLs are 75.76% and 74.77%, respectively. For comparison, blank glass is also shown in figure 1,
and its TL is 74.81%. Results are that the samples prepared with monolayer and bilayer coaings have
almost the same TL as blank glass, showing that as-prepared coatings are transparent.
3.1. Interface structures of monolayer coatings
For sample S, sample AA and sample AAS with monolayer coatings, the cross section SEM images
before and after abrasive testing are shown in figure 2. The water contact angles (WCAs) detected
before and after abrasive testing are also shown in figure 2.
For sample S with a monolayer coating constructed by acidic SiO
2
sol, the interfaces before
(figure 2a) and after (figure 2aʹ) abrasive testing are compact with no voids and holes, showing
strong abrasive resistance. The acidic SiO
2
sol was prepared by the hydrolyed TEOS under acidic
condition, and abundant active hydroxyl groups were originated on the formed silica-based gel
coating [12] and could reacted with hydroxyl groups on glass substrate surface, forming cross-linked
Si-O-Si chemical bonding on the interface. In addition, the WCAs before and after abrasive testing
are 113.34° and 111.07°, also indicating that high wear resistance of the coating constructed by acidic
SiO
2
sol.
For sample AA with a monolayer coating constructed by aggregation of two types of SiO
2
nanoparticles, the interface before (figure 2b) abrasive testing is compact-less with abundant voids
and holes, and after (figure 2bʹ) abrasive testing, there is no coating observed on glass substrate,
showing poor abrasive resistance. The WCA before abrasive testing is 151.53°, and it is significantly
reduced to 101.19° after abrasive testing.
Robust Transparent Super Hydrophobic Coatings and Control of Interface Structure
95
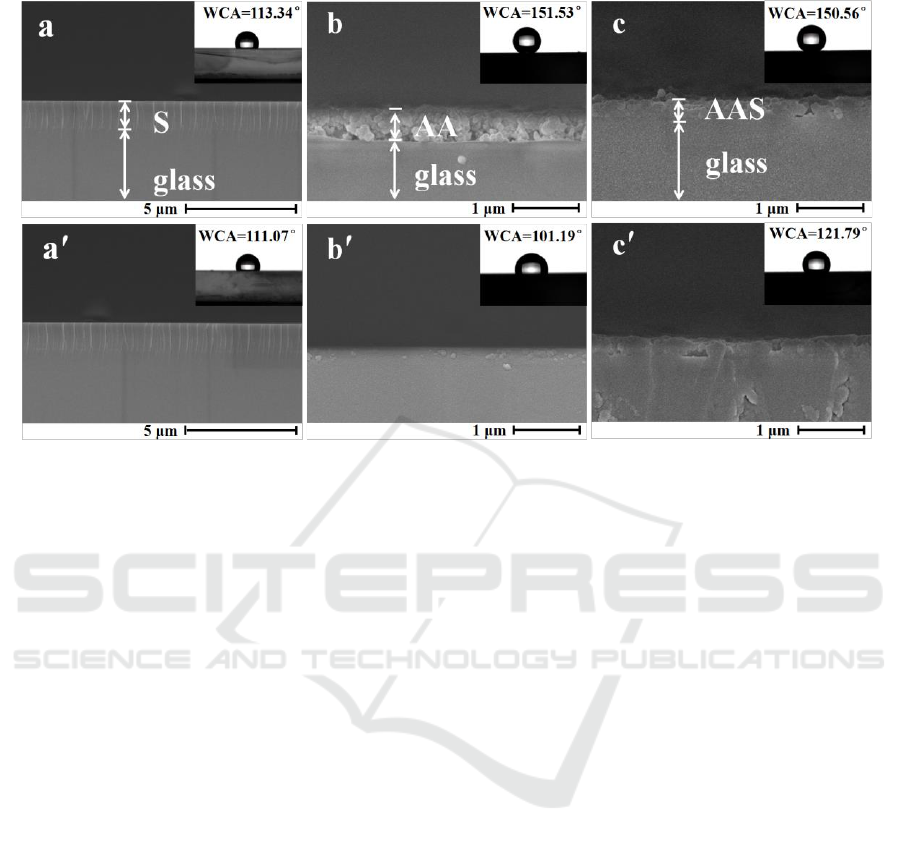
Figure 2.Cross section SEM observations before and after abrasive testing: sample S before (a) and
after (aʹ), sample AA before (b) and after (bʹ), sample AAS before (c) and after (cʹ).
For sample AAS with a monolayer coating constructed by a mixture of SiO
2
nanoparticles and
acidic SiO
2
sol, the compactness of interface before (figure 2c) abrasive testing is improved and there
is less voids and holes on the interface, comparing with the coating constructed only by aggregation
of SiO
2
nanoparticles (sample AA, figure 2b). After abrasive testing (figure 2cʹ), the coating of
sample AAS is only slightly damaged, indicating that the coating has better wear resistance. In
addition, the WCAs before and after abrasive testing are 150.56° and 121.79°, showing mechanical
robustness.
The experimental results of samples with monolayer coatings show that: ①superhydrophobicity
can not be obtained if there is no micro/nano structure (eg. sample S); ②superhydrophobicity can be
achieved when there is micro/nano structure, but if there are abundant voids and holes on interface of
micro/nano structure layer, wear resistance is poor (eg. sample AA); ③reduction of voids and holes
on interface can effectively improve wear resistance and thus maintain good hydrophobicity (eg.
sample AAS). Compactness of interface is vital, and voids and holes on interface of micro/nano
structural coating layer can be controlled by mixing SiO
2
nanoparticles with acid SiO
2
sol, and
mechanically abrasive resistance of micro/nano structure can be enhanced.
3.2. Interface structures of bilayer coatings
For sample S/AA and sample S/AAS with bilayer coatings, the cross section SEM images before and
after abrasive testing are shown in figure 3. The water contact angles (WCAs) detected before and
after abrasive testing are also shown in figure 3.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
96
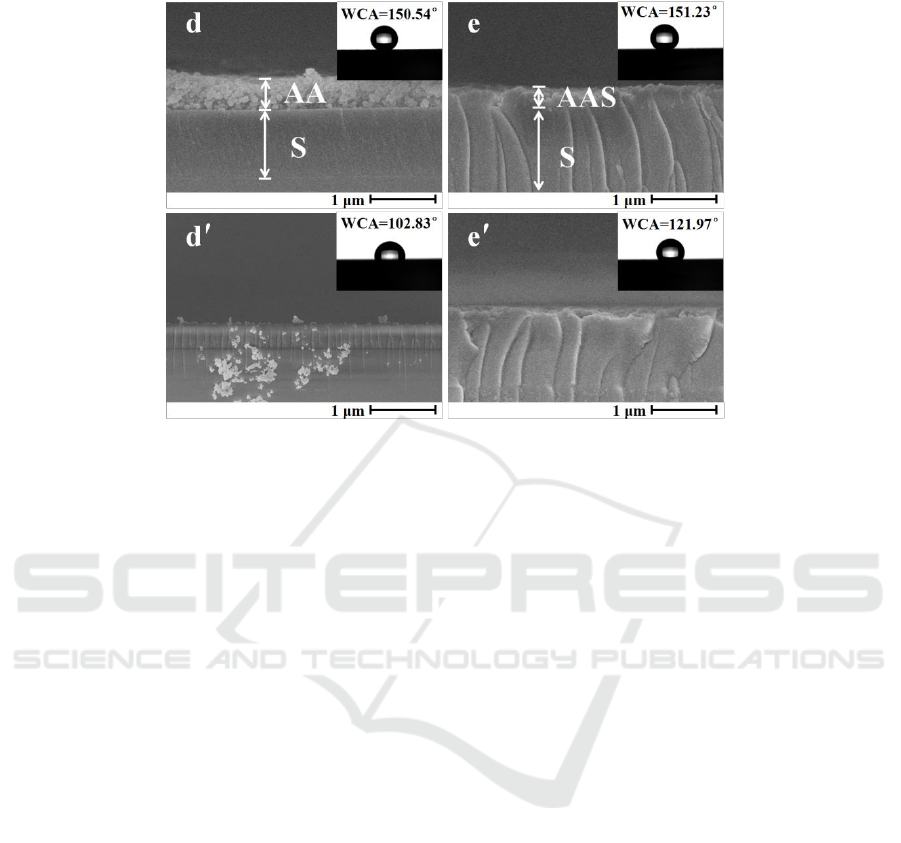
Figure 3.Cross section SEM observations before and after abrasive testing: sample S/AA before (d)
and after (dʹ), sample S/AAS before (e) and after (eʹ).
For sample S/AA with an upper coating constructed by aggregation of two type SiO
2
nanoparticles on an under coating of acidic SiO
2
sol, the upper coating is micro/nano structure before
abrasive testing (figure 3d) but cannot be observed after abrasive testing (figure 3dʹ), showing poor
wear resistance of the upper coating. The WCAs before and after abrasive testing are 150.54° and
102.83°, indicating that the micro/nano structure has poor wear resistance.
For sample S/AAS with an upper coating constructed by a mixture of SiO
2
nanoparticles and
acidic SiO
2
sol on an under coating of acidic SiO
2
sol, the upper coating shows micro/nano structure
before abrasive testing (figure 3e), and the upper micro/nano structural coating is slightly damaged
and still remains observed after abrasive testing (figure 3eʹ). The WCAs before and after abrasive
testing are 151.23° and 121.97°. The reduction of voids and holes on interface of micro/nano
structural coating (figure 3e) results in the improvement of wear resistance of micro/nano structural
coating, which is the same results as samples with monolayer coatings.
3.3. Micro/nano hierarchical surface
Micro/nano hierarchical structure can reduce the contact area between water droplets and solid
surface [1] and is benefit to construct superhydrophobic surface. The surface of sample S/AAS was
characterized by SEM and AFM. figure 4 (a) and (c) are SEM and AFM surface images before
abrasive testing, and figure 4 (b) and (d) are SEM and AFM surface images after abrasive testing,
respectively.
Before abrasive testing, the surface of sample S/AAS is micro/nano hierarchical structure formed
by aggregation of SiO
2
nanoparticles, confirmed by SEM (figure 4a). After abrasive testing, the
micro/nano hierarchical structural surface is damaged to some extent, but the micro/nano structure
still exists (figure 4b). From AFM characterization, the maximum fluctuation and roughness are 340
nm and 40.3 nm before abrasive testing (figure 4c), and decrease to be 158 nm and 20.8 nm (figure
4d), respectively. SEM results well correspond to AFM results.
Robust Transparent Super Hydrophobic Coatings and Control of Interface Structure
97
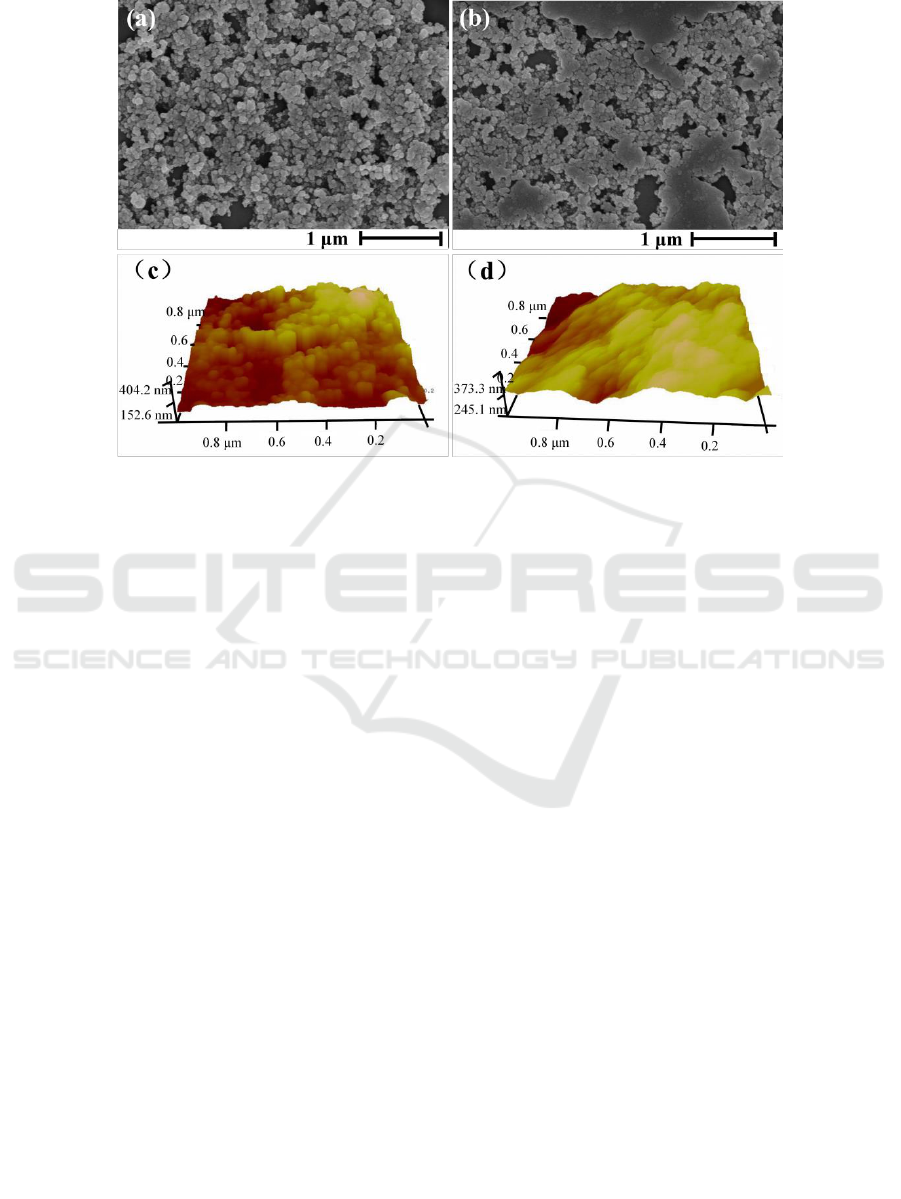
Figure 4.The surface morphology of sample S/AAS. SEM (a) and AFM (c) before abrasive testing;
SEM (b) and AFM (d)after abrasive testing.
4. Conclusions
Abrasive resistance of micro/nano structural coating depends on control of voids and holes on
interface. In this paper, acidic SiO
2
sol was used as a binder, and the interface structure of micro/nano
structural coatings was effectively controlled by mixing process of SiO
2
nanoparticles and the binder.
The voids and holes on the interface were reduced, and mechanically robust transparent
superhydrophobic coating was successfully prepared, showing pratical application prospect.
Acknowledgement
This work is supported by the Science and Technology Major Program of Fujian Province
(2014HZ0005), China.
References
[1] Guo Z G, Liu W M and Su B L 2010 Superhydrophobic surfaces: from natural to biomimetic to
functional J. Journal of Colloid & Interface Science 353(2) 335–55
[2] Li Y, Li X, Guo W J, Wu M C and Sun J Q 2016 Spontaneous wrinkling of layer-by-layer
assembled polyelectrolyte films for humidity-responsive superhydrophobicity J. Science
China Chemistry 59(12) 1–7
[3] Wang N, Xiong D S, Pan S, Deng Y L, Shi Y and Wang K 2016 Superhydrophobic paper with
superior stability against deformations and humidity J. Applied Surface Science 389 354–60
[4] Xu J, Liu Y S, Du W L, Lei W, Si X D, Zhou T, Lin J and Peng L 2017 Superhydrophobic silica
antireflective coatings with high transmittance via one-step sol-gel process J. Thin Solid
Films 631
[5] He Z K, Ma M, Xu X C, Wang J Y, Chen F, Deng H, Wang K, Zhang Q and Fu Q 2012
Fabrication of superhydrophobic coating via a facile and versatile method based on
nanoparticle aggregates J. Applied Surface Science 258(7) 2544–50
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
98

[6] Bayer I S 2017 On the durability and wear resistance of transparent superhydrophobic coatings J.
Coatings 7(12) 7010012
[7] Wang P, Chen M J, Han H L, Fan X L, Liu Q and Wang J F 2016 Transparent and
abrasion-resistant superhydrophobic coating with robust self-cleaning function in either air
or oil J. Journal of Materials Chemistry A 4(20) 7869–74
[8] Wong W S Y, Stachurski Z H, Nisbet D R and Tricoli A 2016 Ultra-durable and transparent
self-cleaning surfaces by large-scale self-assembly of hierarchical interpenetrated polymer
networks J. ACS Applied Materials & Interface 8(21) 13615–23
[9] Ipekci H H, Arkaz H H, Onses M S and Hancer M 2016 Superhydrophobic coatings with
improved mechanical robustness based on polymer brushes J. Surface & Coatings
Technology 299 162–68
[10] Zhi J H and Zhang L Z 2017 Durable superhydrophobic surfaces made by intensely connecting a
bipolar top layer to the substrate with a middle connecting layer J. Scientific Reports 7(1)
9946
[11] Wang C, Tang F, Li Q, Zhang Y and Wang X H 2017 Spray-coated superhydrophobic surfaces
with wear-resistance, drag-reduction and anti-corrosion properties J. Colloids & Surfaces A
Physicochemical & Engineering Aspects 514 236–42
[12] Cai C J, Sang N N, Teng S C, Shen Z G, Guo J, Zhao X H and Guo Z H 2016 Superhydrophobic
surface fabricated by spraying hydrophobic R974 nanoparticles and the drag reduction
in water J.Surface & Coatings Technology 307 366–73
[13] Xu Q F, Wang J N and Sanderson K D 2010 A general approach for superhydrophobic coating
with strong adhesion strength J. Journal of Materials Chemistry 20(28) 5961–66
[14] Shen K B, Yang H, Liu J, Wang W, Huang Y, Zhou Z H and Shen S 2012 Fabrication and
characterization for innate super-hydrophilic SiO
2
thin films J. Materials Science Forum
743–744 377–81
[15] Martin S and Bhushan B 2017 Transparent, wear-resistant, superhydrophobic and
superoleophobic poly(dimethylsiloxane) (PDMS) surfaces J. Journal of Colloid & Interface
Science 488 118
[16] Liu X and He J 2009 One-step hydrothermal creation of hierarchical microstructures toward
superhydrophilic and superhydrophobic surfaces J. Langmuir 25(19) 11822–26
Robust Transparent Super Hydrophobic Coatings and Control of Interface Structure
99
