
Morphological, Dynamic Mechanical, and Mechanical
Properties of Natural Rubber and Poly (Vinyl Alcohol)
Blends
M Jarnthong
*
, K Chen, R Wang, Y P Cao, F Q Zhang, L S Liao and Z Peng
Key Laboratory of Tropical Crop Products Processing of Ministry of Agriculture,
Agriculture Products Processing Research Institute, Chinese Academy of Tropical
Agricultural Sciences, Zhanjiang 524001, China
Corresponding author and e-mail: M Jarnthong, methakarn.jarnthong@yahoo.com
Abstract. Biopolymer blends of natural rubber (NR) late x and poly (vinyl alcohol) (PVA)
were prepared using the latex blending method. The influence of solid content of NR latex on
morphological, dynamic mechanical, mechanical and thermal p roperties of NR/PVA films
was investigated. The results showed that the solid content of NR latex was consistent with
morphology, dynamic mechanical and mechanical properties of the NR/PVA films.
Reduction of NR particle size in NR/PVA blend, increasing trend o f tensile strength and
elongation at break and changing of glass transition temperature ( T
g
) of PVA phase in the
NR/PVA blend were observed with increasing solid content of NR latex.
1. Introduction
Nowadays, the increasing demand of polymer products increases garbage from their wastes, which is
a widely recognized source of pollution. Since most of thermoplastic and elastic materials do not
decompose easily, elimination of their wastes is a serious environmental concern. To minimize this
problem, many researchers have been developed a new material by blending of conventional polymer
with biodegradable polymer [1, 2]. There are variety of biodegradable polymers obtained from
natural product such as starch, cellulose, chitin and chitosan, from microbial fermentation, such as
polyhydroxybutyrate or obtained by chemical synthesis such as polylactic acid, polycaprolactone,
poly (vinyl alcohol), poly (vinyl chloride) and polysaccharides [3-6]. Among these, poly (vinyl
alcohol) (PVA) is one of the most frequently chosen polymers for biodegradable polymer blend
researches [5, 6]. PVA is a non-toxic water-soluble polymer extensively used in paper coating, textile
sizing, flexible water-soluble packaging films and also in food chemistry, pharmaceuticals and
biomedical applications. PVA could be considered as a good host material in polymer blend due to
good thermo-stability, chemical resistance and film forming ability [7].
Natural rubber (NR) is one of important renewable resource elastomers used in many
manufactures, i.e. rubber tires, gloves, condoms and foam products, due to its good elastic properties,
good resilience and damping behavior. Many researchers have been reported blending of natural
rubber with various types of thermoplastic in molten state to improve mechanical properties of
natural rubber including polystyrene, poly(vinyl chloride) and poly(vinyl alcohol) [8-10].
12
Jarnthong, M., Chen, K., Wang, R., Cao, Y., Zhang, F., Liao, L. and Peng, Z.
Morphological, Dynamic Mechanical, and Mechanical Properties of Natural Rubber and Poly (Vinyl Alcohol) Blends.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 12-19
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

Recently, interpenetrating polymer network (IPN) of NR/PVA in latex stage with different types
of crosslinking agents has been studied at various blend compositions for dielectric materials,
bioadhesive and antimicrobial films [11-15]. Improvement of mechanical properties of natural rubber
by increasing PVA content has been reported. However, the study of variation of water
concentrations in the blends has never been reported. In this study, latex stage blending of NR and
PVA at a blend composition of 50/50 w/w was prepared without using of crosslinking agent. The
influence of solid content of NR latex on phase morphology and physical properties of NR/PVA
blends was investigated by complimentary characterization techniques.
2. Experimental
2.1. Materials
Poly (vinyl alcohol) (PVA) (Sigma- Aldrich Inc., Saint Louis, USA) with average molecular weight
of 85,000-124,000 and degree of hydrolysis varying from 87-89% was used for the experiment
without future purification. Centrifuged natural rubber (NR) latex with 60% of dry rubber content
was supplied by Qianjin State Rubber Farm (Zhanjiang, China).
2.2. Preparation of 50/50 NR/PVA blends
The NR latexes at concentrations of 10, 20, 30, 40, 50 and 60 wt% (according to solid content) were
prepared by dilution of centrifuged NRL with de-ionized water. The certain amount of PVA (5 wt%)
was dissolved in de-ionized water and stirred at 65°C for 2 h. The PVA solution was kept at room
temperature for 24 h before use. The 50/50 w/w of NR/PVA blends were prepared by adding NR
latex into the PVA solution and mixed by using magnetic stirrer at room temperature for 30 min. The
NR/PVA latex was cast in glass mold and dried at room temperature for 48 h. After that, the sample
was dried in hot air oven at 50°C for another 48 h.
2.3. Characterization
The viscosity of NR latex was measured by a LVT E3209 Brookfield viscometer (Brook-field
Viscometers Ltd., Harlow, UK) at 25°C.
The transmission electron microscopy (TEM) and scanning electron microscopy (SEM)
techniques were used to visualize the morphology of 50/50 NR/PVA film. For TEM measurement,
the NR/PVA latex samples were diluted and dropped onto carbon coated copper grids to obtain
approximately 60-80 nm thickness film. The micrographs were then recorded using a JEM-1400
TEM (JEOL, Tokyo, Japan) at an accelerated voltage of 80 kV. For SEM measurement, the film
samples were cryogenic fractured in liquid nitrogen and coated the surface by Cu/Pd before
characterized using a Hitachi S-4800 field emission scanning electron microscope (FE-SEM)
(Hitachi, Tokyo, Japan) with an accelerating voltage of 1.0 kV.
Mechanical properties of dried film were analysed by a Hounsfield H10KS universal testing
machine (Hounsfield Test Equipment, Redhill, UK) at room temperature and a crosshead speed of
500 mm/min. The samples were prepared following ASTM D412. The average of five tests was
reported here.
Dynamic mechanical properties of PVA, NR and their blends were determined using a DMTA
8000 (Perkin Elmer Inc., MA, USA). The dual cantilever mode of deformation was used under the
test temperature range from –100 to 100°C with a heating rate of 3°C/min at a frequency of 1 Hz
under liquid nitrogen flow.
Morphological, Dynamic Mechanical, and Mechanical Properties of Natural Rubber and Poly (Vinyl Alcohol) Blends
13
3. Results and discussion
3.1. Viscosity of NR latex
Figure 1 shows the viscosities of NR latexes at various solid contents measured at different rotational
speeds. All samples show shear thinning non-Newtonian rheological behavior. The viscosity of NR
latex increased with increasing solid content, especially at 60 wt%. This is attributed to the increasing
of particles in the system. The viscosity of colloidal solution is a linear function of the volume
fraction of dispersed particles according to Einstein equation [16]:
9
where
r
is the viscosity of the dispersion relative to the viscosity of the solvent and
is the volume
fraction of spherical particles in suspension.
Figure 1. Viscosity of different NR latexes at various rotational speeds.
3.2. Morphology of NR/PVA blends
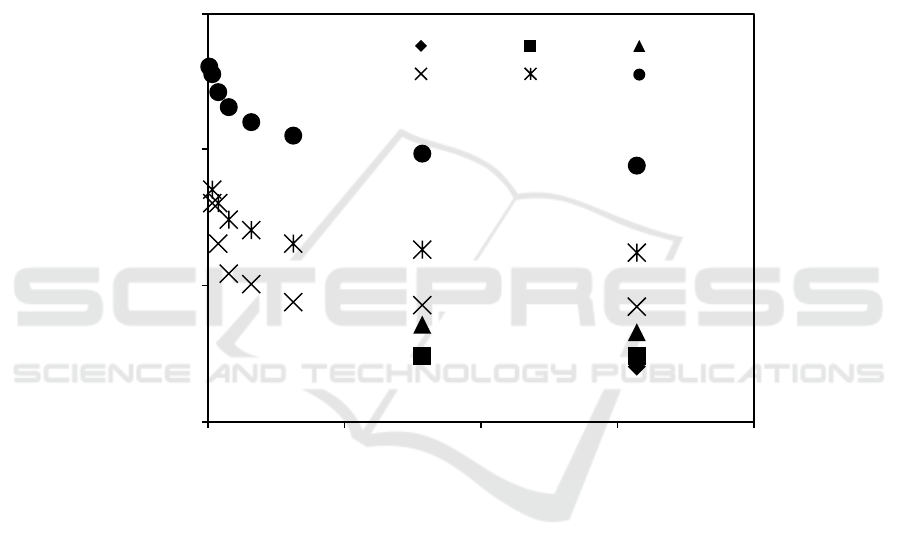
In order to evidence phase morphology and size of NR particles in 50/50 w/w NR/PVA blends, all
samples were examined using TEM and SEM. Figure 2 shows the TEM images of pure 5 wt% PVA
solution and 60 wt% NR latex. It is seen that there was no particle observed in PVA film, while
various sizes of the NR particles were visible as spherical dark spots in the micrograph of NR film.
The TEM micrographs of NR/PVA blends at various solid contents of NR latex are given in
Figure 3. All samples showed the dispersion of dark spots on white background. As compared with
the TEM result of pure polymers in Figure 2., it is indicated that NR particles dispersed in PVA
matrix. The SEM result of fractured surface of NR/PVA blend as shown in Figure 4 also implied the
immiscible between NR and PVA. Particle size of NR in NR/PVA varied from 0.5-2.0 m. Smaller
size of NR particles was observed for the blend obtained from higher solid content of NR latex.
Generally, the stability of colloidal particles is controlled by the balance between repulsive and
attractive forces involved in the system. If the attractive forces, which are assumed to be of London
van der Waals type, are larger than the repulsive forces (i.e., electrostatic, steric, solvation and
1
10
100
1000
0.0 2.0 4.0 6.0 8.0
Viscosity (mPa.s)
Rotational speed (rad/s)
10wt% 20wt% 30wt%
40wt% 50wt% 60wt%
(1)
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
14
Figure 2. TEM micrographs of pure PVA and NR.
Figure 3. TEM micrographs of 50/50 w/w NR/PVA blends at various solid contents of NR latex.
depletion stabilizations), interaction between two or more particles may first cohere to give a loose
aggregate and then subsequently to give a little larger particle [17].
Morphological, Dynamic Mechanical, and Mechanical Properties of Natural Rubber and Poly (Vinyl Alcohol) Blends
15
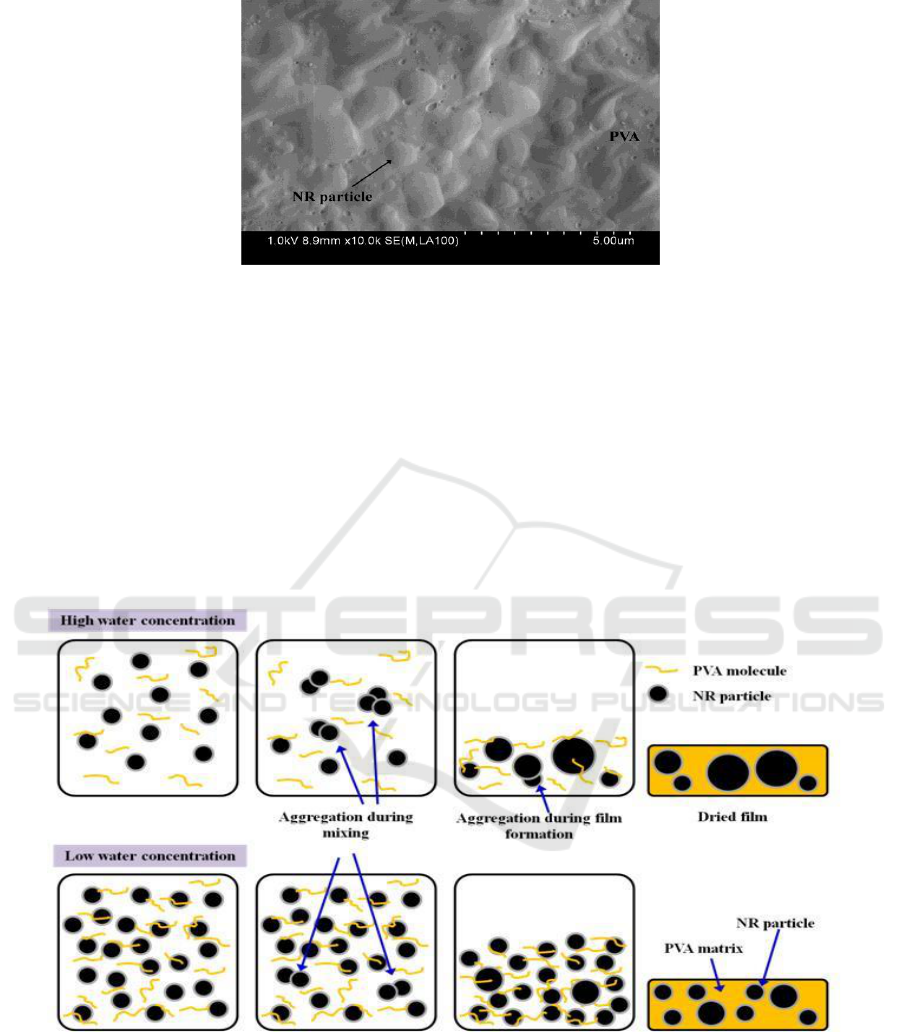
Figure 4. SEM micrograph of fractured surface of 50/50 w/w NR/PVA.
In NR/PVA latex system, the PVA can dissolve well in water medium and act as stabilizer for NR
particles, which hinders the aggregation of NR particles during mixing and film formation. At higher
solid content of NR, the system has higher viscosity and shorter distance between NR particles and
PVA molecules. Therefore, it is possible that the PVA molecules can be adsorbed on NR particles
easier than the NR/PVA system with lower solid content. This caused the increment of repulsive
force between NR particles leading to stabilization of NR particles. In contrast, the dilution of NR
latex by de-ionized water (low solid content of NR) might cause the elimination of repulsive force
between NR particles, which induces to form aggregates of NR particles. This led to the presence of
large NR particles at lower solid content of NR. The proposed aggregation and stabilization of NR
particles by adsorbed PVA molecules during mixing and film formation are depicted in Figure 5.
Figure 5. Schematic diagram of proposed aggregation and stabilization of NR particles during
mixing and film formation.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
16
3.3. Mechanical properties
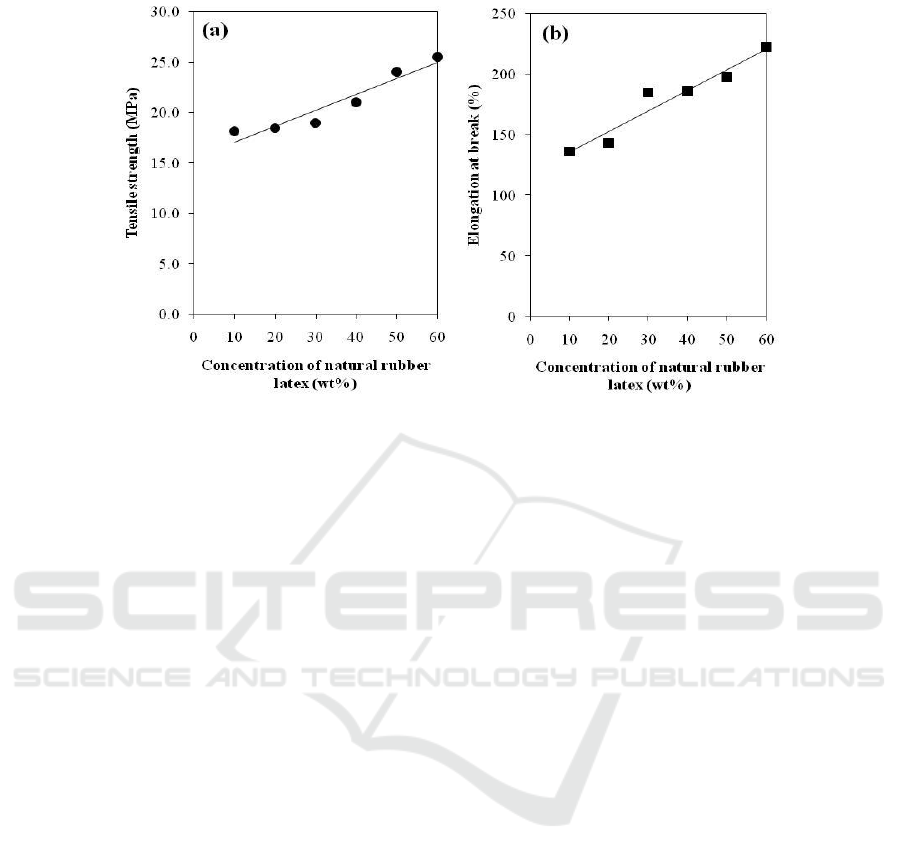
Figure 6. Mechanical properties of 50/50 w/w NR/PVA at various solid contents of NR latex: (a)
tensile strength and (b) elongation at break.
In Figures 6a and 6b, it was found that both tensile strength and elongation at break of NR/PVA
blends showed an increase trend with increasing solid content of NR latex. It can be explained by the
reduction of rubber particle size in the NR/PVA blends, as shown in Figure 3. Decreasing content of
rubber particles in the blend increased interfacial area between dispersed NR and PVA matrix, which
enhanced force transfer between two phases and then provided improvement of mechanical
properties.
3.4. Dynamic mechanical properties
Figure 7 represents the variation of storage modulus (E′) and loss modulus (E″) as a function of
temperature in the range of -100°C to 100°C. In Figure 7a, it is seen that the E' values of PVA were
maximum, while NR showed minimum values. The E' values of the blends were found to be
intermediate between those of pure components. The NR/PVA blends prepared by using 60 wt%
solid content of NR latex showed higher values of E′ compared with those of 30 wt% solid content
indicating higher stiffness of NR/PVA blends.
The influence of temperature on the loss modulus of the samples is shown in Figure 7b. The T
g
was selected as the peak position of E″. The E″ curves of NR/PVA blends clearly appeared two
distinct and separate peaks corresponding to the T
g
’s of NR and PVA. This indicates immiscible
properties between NR and PVA in the NR/PVA blends. However, it is noted that the T
g
’s of NR and
PVA in NR/PVA blends were shifted toward each other implying partially compatible between two
phases [18, 19]. In addition, T
g
of PVA phase in NR/PVA blends shifted toward lower temperature as
the solid content of NR latex increased from 30 wt% to 60 wt%. It might be due to higher surface
area of NR particles at higher solid content of NR latex, which restricted mobility of PVA molecules
and induced greater energy dissipation. This result is in agreement with morphological and
mechanical properties as discussed above.
Morphological, Dynamic Mechanical, and Mechanical Properties of Natural Rubber and Poly (Vinyl Alcohol) Blends
17
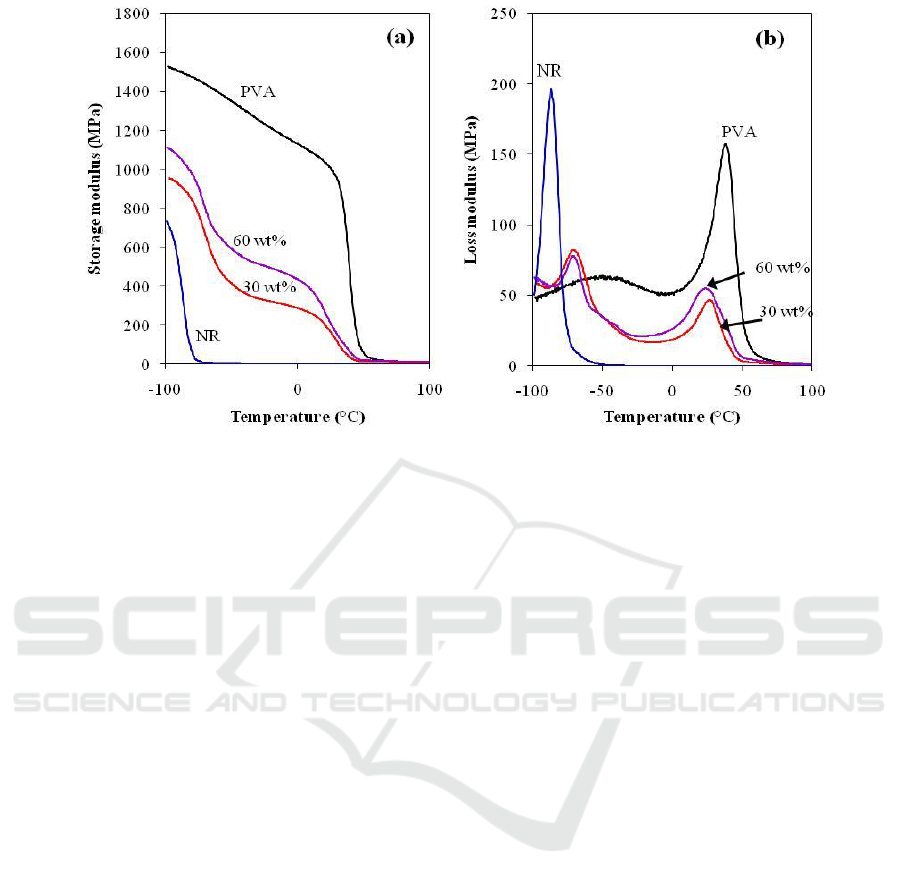
Figure 7. Temperature dependence of (a) storage and (b) loss moduli of PVA, NR and 50/50 w/w
NR/PVA blends.
4. Conclusions
From these results, it can be concluded that the solid content of NR latex showed significant effect on
morphology, mechanical and dynamic mechanical properties of the 50/50 NR/PVA blends.
Increasing solid content of NR latex exhibited reduction of NR particle size in the blends leading to
increase in tensile strength and elongation at break of the blends. Moreover, increasing solid content
of NR latex from 30 wt% to 60 wt% caused increasing of elastic modulus and shifting of T
g
of PVA
phase toward lower temperature indicating the enhanced compatibility between NR and PVA phases.
Acknowledgments
Authors would like to thank the Special Fund for Agro-scientific Research in the Public Interest,
Ministry of Agriculture of the People`s Republic of China (201403066); the earmarked fund for
China Agriculture Research System (CARS-33-JG2); Modern Agricultural Talent Support Program;
National Natural Science Foundation of China (51603230); Major science and technology plan
project of Hainan Province (ZDKJ2016020); Central Public-interest Scientific Institution Basal
Research Fund for Chinese Academy of Tropical Agricultural Sciences (1630122017003) for
financial support.
References
[1] Carvalho A, Job A, Alves N, Curvelo A and Gandini A 2003 Carbohydr. Polym. 53 95-9
[2] Bhatt R, Shah D, Patel K and Trivedi U 2008 Bioresour. Technol. 99 4615-20
[3] Flieger M, Kantorova M, Prell A, Řezanka T and Votruba J 2003 Folia Microbiologica 48 27-
44
[4] Yu L, Dean K and Li L 2006 Prog. Polym. Sci. 31 576-602
[5] Jayasekara R, Harding I, Bowater I, Christie G and Lonergan G T 2004 Polym. Test. 23 17-27
[6] Asran A S, Henning S and Michler G H 2010 Polymer 51 868-76
[7] Anbarasan R, Pandiarajaguru R, Prabhu R, Dhanalakshmi V, Jayalakshmi A, Dhanalakshmi B,
Nisha S U, Gandhi S and Jayalakshmi T 2010 J. Appl. Polym. Sci. 117 2059-68
[8] Arayapranee W, Prasassarakich P and Rempel G 2004 J. Appl. Polym. Sci. 93 1666-72
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
18

[9] Johns J and Nakason C 2012 Polym.-Plast. Technol. Eng. 51 1046-53
[10] Kuriakose B, De S, Bhagawan S, Sivaramkrishnan R and Athithan S 1986 J. Appl. Polym. Sci.
32 5509-21
[11] Jobish J, Charoen N and Praveen P 2012 J. Non-Cryst. Solids 358 1113-19
[12] Riyajan S A, Phupewkeaw N, Maneechay S and Kowalczyk A 2013 Int. J. Adhes. Adhes. 45
84-9
[13] Riyajan S A, Chaiponban S, Chusri S and Voravuthikunchai S P 2012 Rubber Chem. Technol.
85 147-56
[14] Chen K and Wang R 2015 Adv. Mater. Res. 1073 12-15
[15] Jayadevan J, Alex R and Gopalakrishnapanicker U 2017 React. Funct. Polym. 112 22-32
[16] Schneider M, Claverie J, Graillat C and McKenna T 2002 J. Appl. Polym. Sci. 84 1878-96
[17] Liyanage N K 1999 Bull. Rubber Res. Institue of Sri Lanka 40 8
[18] Zhou Y X, Huang Z G, Diao X Q, Weng Y X and Wang Y Z 2015 Polym. Test. 42 17-25
[19] Mazinani S, Darvishmanesh S, Ramezani R and Bruggen B V 2017 React. Funct. Polym. 111
88-101
Morphological, Dynamic Mechanical, and Mechanical Properties of Natural Rubber and Poly (Vinyl Alcohol) Blends
19
