
Hydrochemistry Variations and Carbon Sinks of Cave Stream during
a Storm Event
Xiaoxiao Wang
School of Land and Resources, China West Normal University, Nanchong 637000, China
Email: wxx1989@escience.cn
Key words: Rainfall, cave stream, hydrochemistry, carbon sinks
Abstract: It is a key period to study the hydrochemistry of a cave stream during the storm events. In order to study the
hydrochemistry variations and carbon sinks, the continuous monitoring of a stream in Xueyu cave was
conducted during June 13-15. The hydrochemistry type of the stream was in a type of HCO
3
-Ca. The
geochemistry parameters, such as pH, conductivity, and water temperature, reacted to rainfall quickly. The
response time of hydrochemistry to the rainfall was about 4 hours. Even affected by the piston effect, the
variations for conductivity, HCO
3
-
and Ca
2+
were in negligible magnitudes during a prophase rainfall. The
parameters of conductivity, HCO
3
-
and Ca
2+
declined after the rainfall as a result of the dilution effect, and
the variation of calcite saturation index was consistent with Ca
2+
. The water temperature rose from 16.50 ℃
to 16.58 ℃ due to the calefacient effect of the rainwater. Accompanied with the rise of the water
temperature, the air temperature rose by 0.5℃. The carbon sinks of the studied cave stream were remarkable
during the storm even, and the variations of partial pressure for CO
2
showed a notable increase after the
rainfall. The stock of DIC in the cave stream increased by 15191 kg, and the absorbed CO
2
was 5479 kg
during the storm event. Therefore, the role of cave stream in the carbon sinks should be paid more
attentions.
1 INTRODUCTION
The karst area in Southwest China is about 53 km
2
,
it is the largest karst region in the world (Pu et al.,
2010). As crannies and conduits well develop in this
area, the cave streams are important for water supply
in local area (Yuan, 2000). Due to the duality of
water storage in surface ground and underground,
the transform of surface and underground water is
very quickly, especially during a storm event (Yang
et al., 2012). With the obviously global climate
change, the carbon sink caused by karst processes
has been paid more attentions
(Yuan, 2011). As we
all know, the carbon sink could be driven by the
formation of carbonate rocks in a long-time scale in
the geologic history (Cao et al., 2011). The karst
processes can happen in the normal atmospheric
temperature with an open system, which is sensitive
to the environment changing (Li et al., 2004; Zhang
et al., 2005; Liu et al., 2005; Liu and Yuan, 2000).
Therefore, it is important to study the karst process
in a short-time scale, especially during a storm event
(Liu and Zhao, 2000). Rainfall period is an
important period to study the karst process, as we
can monitor the response of the karst processes to
the environment change (Liu et al., 2007). In this
paper, a three days’ monitoring of a cave stream was
carried out during a storm event, and the objects of
the study are: 1) to study the variations of
hydrochemistry of the cave stream, and 2) to
evaluate the carbon sinks of the cave stream during
the storm event.
2 STUDY AREA
Xueyu cave (29°47′ N, 107°47′ E) is located in
Fengdu county, Chongqing, China. It is on the bank
of Long River, a branch of Yangtze River. It is about
16 km far away from the downtown of Fengdu. The
altitude of the Xueyu cave entrance is about 233 m,
which is about 55 m above water level of the Long
River. The cave is developed in the Fangdoushan
anticline, which is located in the paralleled
ridge-valley area of east Sichuan. The cave follows
the strike of the stratum, and the length of the cave is
1643 m. There are three floors in the cave, and the
underground river is developed in the lowest floor,
288
Wang, X.
Hydrochemistry Variations and Carbon Sinks of Cave Stream during a Storm Event.
In Proceedings of the International Workshop on Environment and Geoscience (IWEG 2018), pages 288-293
ISBN: 978-989-758-342-1
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
of which the water discharges to the Long river (Zhu
et al., 2004). The temperature of the cave is in the
ranges of 16-18 ℃, and temperature variations exist
among different floors. The humidity is above 95%
all the year round, and it is 100%in the floor with the
stream flowing (Wang, 2010). With the subtropical
monsoon climate, the mean annual precipitation of
the study area is 1072 mm. Affected by the
Southwest and Southeast monsoon, most of the
rainfall is in April to September. The thickness of the
overlying rock is 150 to 250 m, and the thickness of
overlying soil is 0 to 90 cm. The local vegetation is
evergreen broad-leaved forest and shrub (Pu et al.,
2009).
3 METHODS
The data of water temperature, water stage, pH and
conductivity from 13 June to 15 June, 2011 was
collected from a Greenspan CTDP 300
multi-channel data logger, which was placed in the
underground stream near the cave entrance. Water
stage, water temperature, pH and electrical
conductivity (Ec) were monitored every 15 minutes.
The discharge was calculated by the model of
Rectangular Weir at the cave entrance. The cave air
temperature was obtained by the OM-EL-USB-2
multi-recorder, which is placed at the second floor of
the cave, with the measurement range of -35~80 ℃
and accuracy of 1℃. In order to get the weather
information, a Davis BS28-VP2 mini weather station
was placed on a roof, which is about 500 m to the
cave entrance. The wind speed, wind direction, air
temperature, relative humidity, air pressure, and
rainfall were recorded by the mini weather station.
The samples of the rain water were collected during
the study from 13 to 15 June, and the stream water
samples were collected every month in 2011. All the
water samples were collected by cleaned polythene
bottles and acidified with 1:1 nitric acid. Cations of
the water samples were measured in the
geochemistry and isotopic laboratory of Southwest
University by Perkin-Elmer Optima 2100DV
ICP-OES, with accuracy of 0.001mg/L. Anions of
the water samples were measured by Ion
Chromatograph, with the accuracy of 1 ppb. The
concentration of HCO
3
-
was determined by the
Aquamerck Alkalinity Test, with the accuracy of 0.1
mmol/L. The saturation index of calcite (SIc) and
partial pressure of CO
2
(Pco
2
) were calculated by
WATSPEC software.
4 RESULTS AND DISCUSSION
4.1 Hydrochemistry Variations of the
Cave Stream
To study the hydrochemistry variations of the stream,
the water samples were monitored every month in
2011. The equivalents per hundred of Ca
2+
was
90.55%, it was 66.01% of HCO
3
-
and 17.26% of Cl
-
..
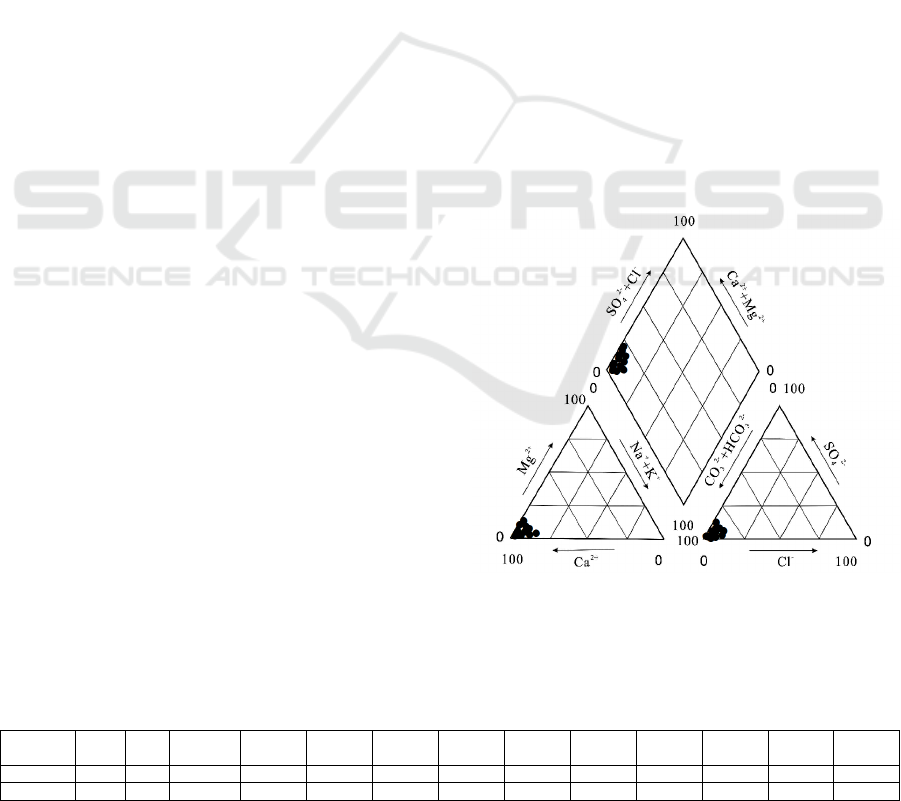
Data of the cations congregated on the side of Ca
2+
,
and the anions assembled on the side of HCO
3
-
(Figure 1). Therefore, the hydrochemistry type of the
stream water was HCO
3
-Ca. The ratio of
(Ca
2+
+Mg
2+
)/HCO
3
-
was 0.48, which was close to
0.5 (Xiao et al., 2012). So, the weathering type of
the drainage area for the cave stream was dominated
by carbonate rock weathering.
Figure 1: Hydrochemistry type of the underground water
in Xueyu cave.
Table 1: Hydrochemistry of rain water and stream water (13~15 June, 2011)
Water
type
T
w
/℃
pH EC/
(μS·cm
-1
)
Ca
2+
/
(mg·L
-1
)
Mg
2+
/
(mg·L
-1
)
Na
+
/
(mg·L
-1
)
K
+
/
(mg·L
-1
)
HCO
3
-
/
(mg·L
-1
)
Cl
-
/
(mg·L
-1
)
SO
4
2-
/
(mg·L
-1
)
Sr
+
/
(mg·L
-1
)
Si
+
/
(mg·L
-1
)
NO
3
-
/
(mg·L
-1
)
Rain 24.2 5.76 92 6.315 0.151 0.111 0.188 6.1 7.373 4.846 0.015 0.123 2.159
Stream 16.5 7.62 405 102.213 2.395 0.985 0.473 219.6 16.023 16.503 0.831 3.775 8.025
Hydrochemistry Variations and Carbon Sinks of Cave Stream during a Storm Event
289
4.2 Rainfall’S Effects on the
Hydrochemistry Variations of the
Cave Stream during a Storm Event
4.2.1 Hydrochemistry of Rainwater
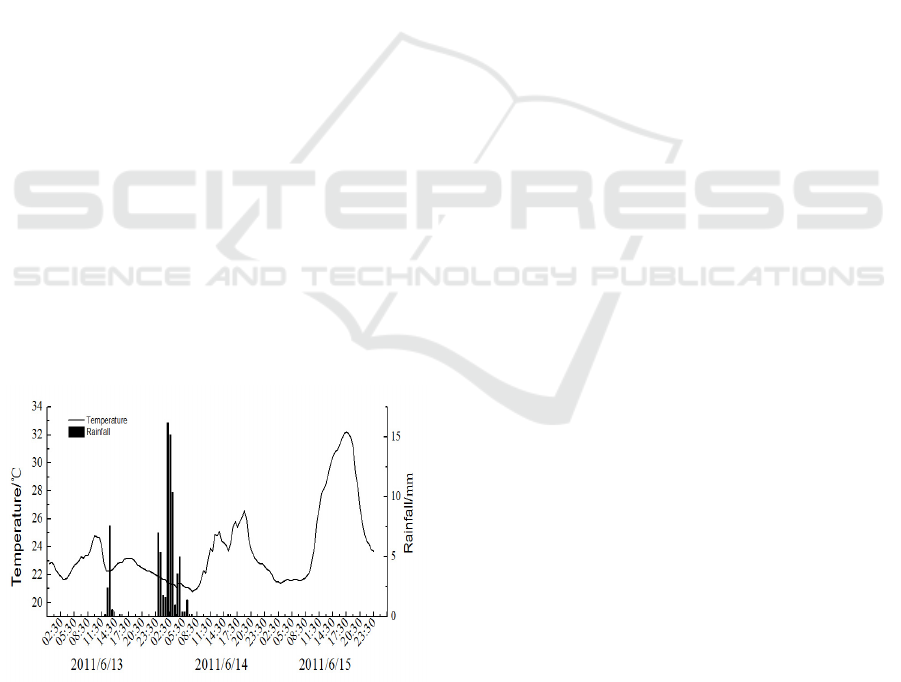
There were two rainfall periods during the study
from 13-15June. The first period was from 11:30 to
14:30 June 13, and the precipitation was 11 mm. The
second phase was from 22:30, 13 June to 5:30, 14
June, and the precipitation was 69.6 mm. The total
precipitation was 80.6 during the study (Figure 2).
The temperature was about 25℃ before the first
rainfall, but it dropped to 22℃ during the first rainfall
event. The pH of the rain water was 5.76, which was
closed to 5.6, the pH of acid rain. The acid rain in
Chongqing area was featured by high concentrations
of Cl
-
, NO
3
-
, and SO
4
2
(Chen et al., 2012)-. As Cl
-
is
steady in the hydrological cycle and is little affected
by human activities (Meybeck, 1979) Therefore, Cl
-
was an important parameter to evaluate the rainfall’s
effects on the variations of surface water
hydrochemistry (Grosbois et al., 2000). The ratio of
Na
+
/Cl
-
in sea water was 0.86 (Meybeck, 1979), but
the ratio for rain water during 13-15 June was 0.015,
which indicated the rain water was merely affected
by sea water. The temperature of rain water was
higher than that of the cave stream water (Table 1),
therefore, the stream water could be heated by the
rain water. The concentrations of Ca
2+
and Mg
2+
in
rain water were lower compared with those of the
stream water, so the hydrochemistry of the stream
water would be lightly affected by the rainfall.
Figure 2: The variations of rainfall precipitation and air
temperature from June 13 to June 15, 2011.
4.2.2 The Hydrochemistry Variations of the
Cave Stream Water
The data of water temperature, pH and Ec could
get from the CDTP 300 multi-channel data
logger
during the rainfall period. The coefficient R
2
between Ca
2+
and EC was 0.732, and it was 0.856
between HCO
3
-
and EC. Therefore, the
concentrations of Ca
2+
and HCO
3
-
could be
calculated by the Formula 1 & 2.
[Ca
2+
]=0.209·Ec+5.454 (1)
[HCO
3
-
]=0.751·Ec - 62.75 (2)
The first rainfall didn’t cause the hydrochemistry
variations of the stream water due to the little
precipitation (Figure 3). The water in the soil was
saturated after the first rainfall, and the coming
rainfall in the morning of 13 June could become
overland flow, which would flow into the stream
quickly. Affected by the rainfall, the discharge of the
stream reached the peak at 3:00, 13 June. The time
lag between the rainfall and the highest discharge
was about 4 hours. The pH of the stream water was
7.93 before the second rainfall, but it dropped to
7.65 after the rain water pouring into the stream. The
reasons for the pH dropping were: the dissolved CO
2
in the stream water, and mixture of the stream water
and the rain water with low pH. The concentrations
of HCO
3
-
, Ca
2+
and Ec in the stream water showed
no obvious variations before the second rainfall.
However, the concentrations of HCO
3
-
, Ca
2+
and Ec
rose markedly during the second rainfall, which was
attributed to the old water in the soil and cranny was
pushed out by rain water. Dilution effect dominated
the variations of the hydrochemistry of the stream
water after the rainfall, and the concentrations of
HCO
3
-
, Ca
2+
and Ec decreased. When the rainfall
came to maximum, the saturation index of calcite in
the stream water was above zero, which indicated
the calcite in the water was saturated and the erosion
ability of the stream water was weak. The variations
of the saturation index of calcite was consistent with
HCO
3
-
, Ca
2+
and Ec, which was mainly affected by
old water in the soil and cranny.
4.2.3 Rainfall’s Effects on the Temperature
of the Cave Air and the Stream Water
In summer, the temperature of the atmosphere is
higher than that of the cave stream water (Yang et al.,
2009). The rain water will be heated by atmosphere,
soil and vegetation when flowing on the ground
IWEG 2018 - International Workshop on Environment and Geoscience
290
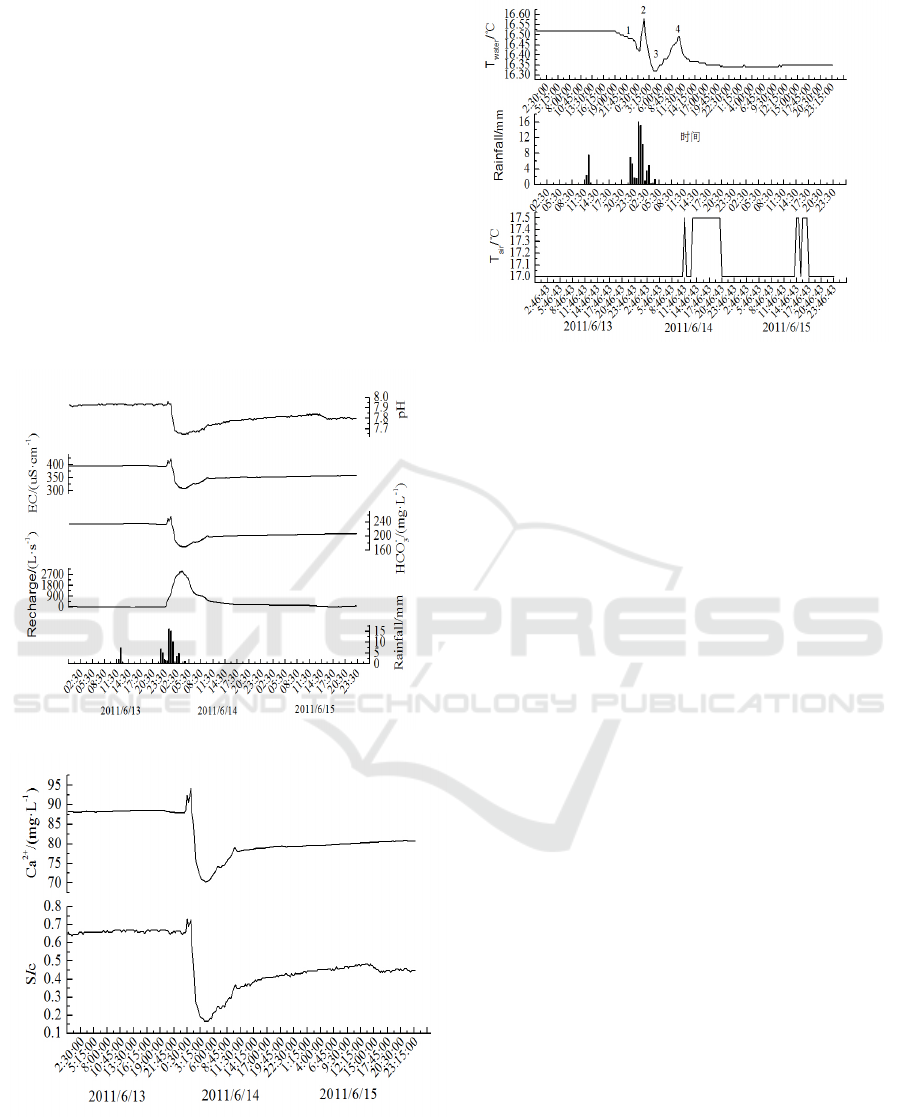
surface. The first rainfall was not adequate to form
surficial runoff, and the stream water was little
affected by the first rainfall. The temperature of the
atmosphere decreased in the night of June 13 (Figure
3), which could influence the water’s temperature
(Figure 4). With the precipitation of the rainfall
becoming larger in the morning on June 14, the
heated rain water poured into the cave stream, and
the temperature of the stream water rose slowly,
reaching a peak value of 16.58 ℃ at 2 o’clock, June
14 (Figure 4). As a result of lacking heat to make the
temperature of the stream water get higher, the
temperature of the stream water became lower
(Figure 4). After the air’s temperature became higher
in the afternoon on June 14, the stream water’s
temperature increased slowly.
Figure 3: Variations of pH, EC, HCO3-, Ca2+, discharge,
and SIc of stream water from June 13 to June 15, 2011.
Figure 4: Variations of water temperature and air
temperature in Xueyu cave from June 13 to June 15, 2011.
Previous studies showed that the variations of
cave air’s temperature related to the climate change,
altitude, ventilation, rainfall event (Stoeva et al.,
2006). Besides, cave air’s temperature can vary in
periods of 24 hours and 12 hours due to the
variations of the atmosphere’s temperature (Sondag
et al., 2003). With three floors and only one entrance
in Xueyu cave, the ventilation of the cave was
indistinctively. There was no fluctuation in the
temperature of the cave air during no rain period
(Figure 4), which indicated the temperature of the
cave air was stable in day and night. However, the
stream water’s temperature increased after the
second rainfall (Figure 4). The cave air’s
temperature rose to 17.5 °C 10 hours after the
rainfall, and the cave air’s temperature dropped to
17.0 °C at 20:00 on June 14, and rose to 17.5 °C
again at 14:00 on June 15. It was about 10 hours
from the rise of stream water’s temperature to the
rise of cave air’s temperature. Therefore, the rainfall
affected the variations of the cave air’s temperature,
which followed the rise of the stream water’s
temperature.
4.3 Carbon Sinks in the Stream Water
during Rainfall Event
Dissolved inorganic carbon (DIC) in stream water
comes from Karst process by absorbing CO
2
and the
weathering of carbonate rock (Jiang, 2000). Some
studies found that dissolved CO
2
in the water was
more stable than we thought (Adamczk et al., 2009),
which made the carbon sinks can be evaluated
during the rainfall periods. HCO
3
-
is the main form
of DIC in the water when pH is below 8.2 (Cao,
2012). The pH of the stream water during the rainfall
Hydrochemistry Variations and Carbon Sinks of Cave Stream during a Storm Event
291
period from June 13 to June 15 was below 8.0,
therefore, the amount of DIC was equal to the
amount of HCO
3
-
in the stream water.
Fllowing the reaction equation of the karst
processes:
Limestone:
CaCO
3
+CO
2
+H
2
O — Ca
2+
+2HCO
3
-
(3)
Dolomite:
CaMg(CO
3
)
2
+2CO
2
+2H
2
O — Ca
2 +
+Mg
2+
+4HCO
3
-
(4)
The absorbed CO
2
in the underground water can be
calculated:
The amount of absorbed CO
2
(mg/s) =
1/2[HCO
3
-
]×44×Q (Liu, 2000) (5)
([HCO
3
-
] is the concentration of HCO
3
-
; Q is the
discharge of the underground water.)
The amount of DIC (mg/s) = concentration of DIC
×Q (6)
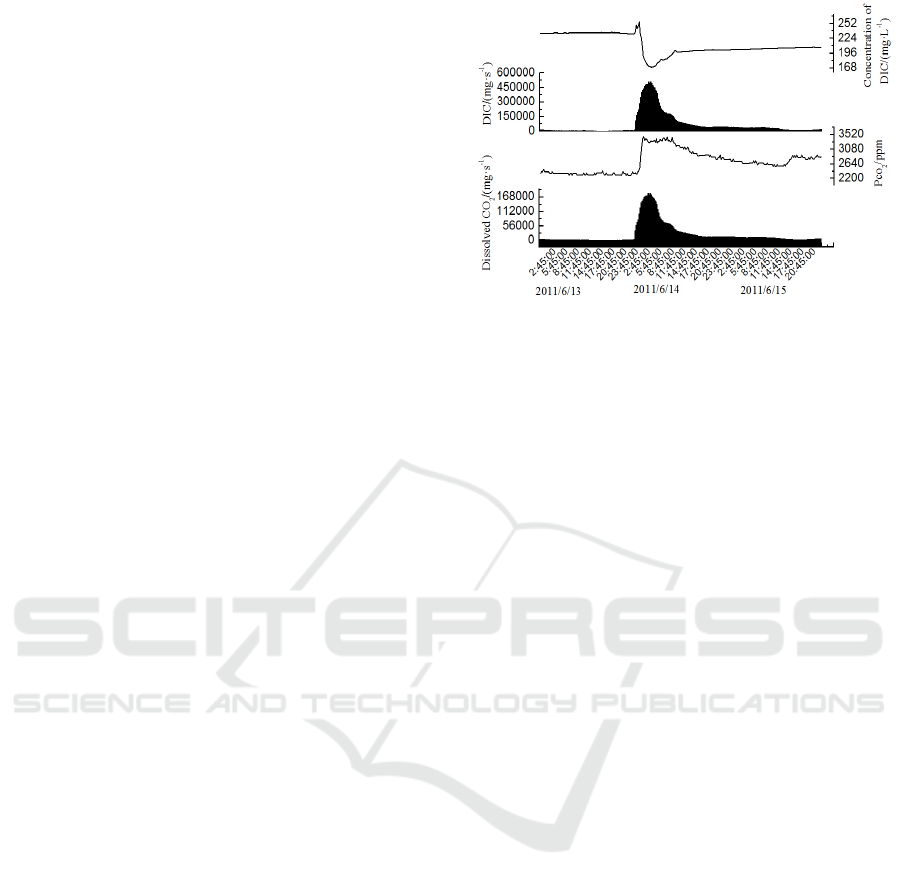
The concentration of DIC increased firstly and
then declined to the minimum value when the
rainfall reached the maximum (Figure 5). With old
water pouring into the stream, the concentration of
DIC increased in the prophase of the rainfall.
However, the concentration of DIC decreased, which
was affected by the dilution effect of the rainfall.
Though the concentration of DIC decreased, the
amount of DIC in the stream increased due to the
increase of the discharge, rising from 15.0 g/s to 511
g/s. The amount of absorbed CO
2
increased with the
increase of discharge, from 5.94 mg/s to 184 mg/s.
The variations of the partial pressure of CO
2
indicated that the partial pressure of CO
2
increased
with more CO
2
dissolved in the stream water.
The total amount of dissolved CO
2
during the
rainfall period was 6202 kg, and total amount of
DIC was 17198 kg. The amount of dissolved CO
2
increased by 5479 kg, and the amount of DIC
increased by 15191 kg compared with those of
pre-rainfall period. The reason for the increase of
dissolved CO
2
and DIC were that the increase of the
discharge of the stream and the erosion of carbonate
rocks.
Figure 5: Variations of Pco2, absorbed CO2 and DIC
of stream water from June 13 to June 15, 2011.
5 CONCLUSIONS
The hydrochemistry type of the cave stream water is
HCO
3
-Ca, and the weathering type in the study area
carbonate weathering. The hydrochemistry of the
stream is merely affected by the rainfall. Piston
effect and dilution effect dominated the variations of
the hydrochemistry of the stream.
The discharge of the stream increased after the
rainfall during the study. The decrease of the pH
during the rainfall was due to the CO
2
effect and the
mixture of rain water. Affected by the piston effect,
the concentrations of HCO
3
-
and Ca
2+
, and Ec all
showed increase trends during the early rainfall
period. However, the dilution effect dominated the
decrease of HCO
3
-
, Ca
2+
, and EC after the rainfall
became heavier, and the saturation index of calcite
also showed a decrease trend. The temperature of the
stream water increased during the rainfall period,
and the response time of the stream was 4 hours.
Cave air’s temperature was also increased after the
rainfall, and the response time was 10 to 28 hours.
The amount of DIC was 17198 kg transported
by the stream water during the study, and the amount
of dissolved CO
2
in the water was 6202 kg. The
amount of dissolved CO
2
increased by 5479 kg, and
the amount of DIC increased by 15191 kg compared
with those of pre-rainfall period.
ACKNOWLEDGEMENT
This study was supported by the Start-up funds for
doctoral research of China West Normal University
(412654).
IWEG 2018 - International Workshop on Environment and Geoscience
292

REFERENCES
Adamczk K, Schwarz M P, Pines D 2009 Real-Time
Observation of Carbonic Acid Formation in Aqueous
Solution. Science 326 1690-1694
Cao J H, Yang H, Kang Z Q 2011 The calculation of
carbon sinks of carbonate erosion: Taking Pearl River
for example. Chinese Science Bulletin 56(26)
2181-2187
Cao M 2012 Effects of urbanization on hydrogeochemical
and stable isotopic characteristics of karst
groundwater. Master thesis
Chen H L, Li T Y, Zhou F L 2012 Analysis on chemical
characteristics of meteoric precipitation based on the
data of a series of rainwater—a case study from the
Southwest University, Beibei district, Chongqing.
Journal of Southwest University(Natural Science
Edition) 34(2) 105-113
Grosbois C, Negrel P, Fouillac C 2000 Dissolved load of
the Loire River: chemical and isotopic
characterization. Chemical Geology 170 179-201
Jiang Z C 2000 Carbon cycle and ecological effects in
epikarst systems in Southern China. Quaternary
sciences 20(4) 316-323
Li W, Yu L J, Yuan D X 2004 Bateria biomas sand
carbonic anhydrase activity in some karst areas of
southwest China. Journal of Asian Earth Sciences 24
145-152
Liu Z H, Li Q, Sun H L 2005 Diurnal variations in
hydrochemistry in a travertine2depositing stream at
Baishuitai , Yunnan , SW China : Observations and
Explanations. Hydrogeology & Engineering Geology
6 10-15
Liu Z H, Li Q, Sun H L 2007 Seasonal, diurnal and
storm-scale hydrochemical variations of typical
epikarst springs in subtropical karst areas of SW
China: Soil CO
2
and dilution effects. Journal of
Hydrology 337 207-223
Liu Z H, Yuan D X 2000 Features of Geochemical
Variations in Typical Epikarst Systems of China and
Their Environmental Significance. Geological Review
46(3) 324-327
Liu Z H. 2000 Contribution of carbonate rock weathering
to the atmospheric CO
2
sink. Carsologica Sinica 19(1)
293-300
Liu Z, Zhao J 2000 Contribution of carbonate rock
weathering to the atmospheric CO
2
sink.
Environmental Geology 39 1053-1058
Meybeck M 1979 Concentrations des eaux fluviales en
elements majeurs et apports en solution aux oceans.
Rev Geol Dyn Geogr Physs 21 215-246
Pu J B, Shen L C, Wang A Y 2009 Space-time variation of
hydro-geochemistry index of the Xueyu cave system
in Fengdu county, Chongqing. Carsologica Sinica
28(1) 49-54
Pu J B, Yuan D X, Jiang Y J 2010 Hydrogeochemistry and
environmental meaning of Chongqing subterranean
karst streams in China. Advances in Water Science
21(5) 628-636
Sondag F, Ruymbeke M V, Soubie S F 2003 Monitoring
present day climatic conditions in tropical caves using
an Environmental Data Acquisition System (EDAS).
Journal of Hydrology 273 103-118
Stoeva P, Stoev A, Kiskinova N 2006 Long-term changes
in the cave atmosphere air temperature as a result of
periodic heliophysical processes. Physics and
Chemistry of the Earth 31 123-128
Wang A Y 2010 Study on operation regularity and
environmental information reservation of Cave Karst
Dynamic System. Master thesis
Xiao Q, Shen L C, Yang L 2012 Weathering seasonal
variations in karst valley in Southwest China.
Environmental Science 33(4) 1122-1128
Yang P H, Liu Z Q, He Q F 2012 Transportation and
sources of the suspended particle in a karst spring
during a storm event. Environmental Science 33(10)
3376-3381
Yang P H, Luo J Y, Yuan D X 2009 Response of spring
water hydrochemical behaviors to rainfall in karst
subterranean river system. Journal of Hydraulic
Engineering 40(1) 67-74
Yuan D X 2000 Aspects on the new round land and
resources survey in karst rock desertification areas of
South China. Carsologica Sinica 19(2) 103-107
Yuan D X 2011 The review and prospect of research
about geology and carbon cycle. Chinese Science
Bulletin 56(26) 2157-2157
Zhang C, Yuan D X, Cao J H 2005 Analysis on the
environmental sensitivities of typical dynamic epikarst
system at the Nongla monitoring site. Environmental
Geology 47(5) 615-619
Zhu X W, Zhang Y H, Han D S 2004 Cave characteristics
and speleothems in Xueyu cave group, fengdu,
Chongqing city. Carsologica Sinica 23(2) 85-89
Hydrochemistry Variations and Carbon Sinks of Cave Stream during a Storm Event
293
