
Species Diversity and Distribution of Scleractinian Coral at Daao
Bay, Shenzhen
Fei Tong, Lu Zhang, Pimao Chen
*
and Wenjin Chen
South China Sea Fisheries Research Institute Shenzhen test base. South China Sea Fisheries Research Institute, Chinese
Academy of Fishery Sciences. Scientific Observing and Experimental Station of South China Sea Fishery Resources and
Environment, Ministry of Agriculture, P. R. Guangzhou, Guangdong, 510300, China
Email: chenpm@scsfri.ac.cn
Keywords: Scleractinian, species diversity, distribution
Abstract: Species diversity and distribution status of the scleractinian were surveyed by Line Intercept Transect
method at Daao Bay in the east Shenzhen in 2017. Through the image survey data and sample analysis to
filed survey, this survey got 13 kinds of scleractinian coral, which the main dominant species is Platygyra
yaeyamaensis. Statistical analysis showed that Daao Bay scleractinian coral coverage rate was 9.12 %, the
Shannon-wiener diversity index, Simpson’s diversity index and Margarlef species richness index were
2.251 nit, 1.754 and 0.856, respectively. The scleractinian coral coverage rate declined seriously compared
to 2007. The coral communities were experiencing degradation. Fortunately, there was 0 bleaching or dead
scleractinian coral coverage found in this vessel. This area was disturbed highly by human activities, which
may alter the natural disturbance regimes of coral reefs by transforming pulse events into persistent
disturbance or even chronic stress, by introducing new disturbance, or by suppressing or removing
disturbance. It should strengthen monitor coral reef and its ecosystem in this area for better protecting
increasingly recession coral reef resources.
1 INTRODUCTION
Coral reefs, mangroves and sea grass beds are
important three typical marine ecosystems, with
high biodiversity and primary productivity
(Muruganantham et al., 2017; Halik and Verweij,
2017;
De et al.2018), providing rich food and
habitats for marine life (Roelfsema et al., 2018; Eyal
et al., 2015). It also provides mankind with a great
deal of material products and extremely high
aesthetic value (Moberg and Folke 1999). But the
community structure, composition and ecosystem
functioning of coral reefs have been extensive
changed by the human’s disturbances
(Ferrigno et
al., 2016). Coral is sensitive to environmental
changes (
Holden and Ledrew 2002). When it suffers
external environmental pressure beyond physical
tolerance, it will release the symbiotic zooxanthellae,
resulting in coral bleaching (Glynn 1993). Facing
the human-dominated world, ecologists are now
reconsidering the role of disturbance for coral reef
ecosystem. Human activities alter the natural
disturbance regimes of coral reefs by transforming
pulse events into persistent disturbance or even
chronic stress, by introducing new disturbance, or by
suppressing or removing disturbance. Which has
caused widespread concern around the world
(Nyström et al., 2000 . The coral reef monitoring
network has been established worldwide to monitor
the health status of coral reef ecosystems which
would contribute to prevent the degradation of
valuable coral reef resources (Tun and Wilkinson
2004). Understanding the species distribution and
diversity of corals is an important part of coral
conservation, Mebrahtu investigated variation in
scleractinian coral Richness and community
diversity in the Western Indian Ocean on different
spatial scales to understand how diversity is
organized is essential to inference about appropriate
scales for natural resource research and management
(Ateweberhan and Mcclanahan 2016). Hector
considered sampling scale and lack of attention to
taxa other than scleractinian corals have limited the
capacity to protect coral reefs and coral communities
50
Tong, F., Zhang, L., Chen, P. and Chen, W.
Species Diversity and Distribution of Scleractinian Coral at Daao Bay, Shenzhen.
In Proceedings of the International Workshop on Environment and Geoscience (IWEG 2018), pages 50-55
ISBN: 978-989-758-342-1
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
(Guevara and Breedy 2004). Alessandro Cau using a
combination of multivariate statistical analyses
reveal that environmental and bathymetric factors
were important drivers of the observed patterns of
coral biodiversity (Cau et al., 2017). Oktiyas
Muzaky Luthfi use Line Intercept Transect to assess
the condition and distribution of stony corals at
Karang Pakiman Reef, Bawean Islands
(Luthfi and
Anugrah 2017). Samantha Howlett data on live coral
cover, coral genus, diversity, and coral colony
structure type to compared to give an indication of
reef quality between habitats (Howlett et al., 2016).
Daao Bay was a good habitat for scleractinian
corals before. As the economy developed rapidly in
recent years, many real estate and tourism projects
had been developed in the near shore. The
distribution and health status of coral resources is
facing disturbance, this study using Line Intercept
Transect to study the diversity, distribution and
abundance of Scleractinian coral in Daao Bay.
Which could assess the health condition of coral reef
as a basic ecosystems for reference conservation.
2 MATERIALS AND METHODS
2.1 Research Area
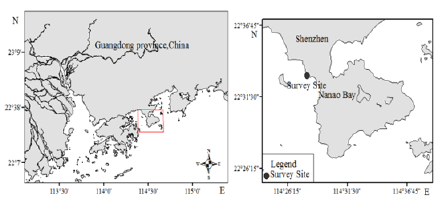
The research was conducted along the Daao Bay,
Shenzhen in November 2017. Which located at the
Daao Bay Coast (114°28′02.10″E, 22°33′06.21″N
~114°27 ' 58.52 "E, 22°33 ' 10.59" N) (Fig. 1). The
scleractinian coral species distribution, health and
sediment conditions were evaluated in this coral reef
area. The survey water bottom sea temperature was
22.5℃, transparency was 2.5 m and salinity was
32.2‰. With handheld GPS Positioning System
(78s, Garmin, American) determines the exact
position in this investigation. Investigate sea water
depth was range of 3-10 meters.
Figure 1: Scleractinian coral survey stations
2.2 Survey Methods
In this area, the survey and sampling of coral reefs
are an international commonly used Line Intercept
Transect (Luthfi and Anugrah 2017, Amin, 2017,
Facon et al., 2016). Fifty meter long transect were
laid in this zonation and parallels to the shoreline,
date and samples were got by SCUBA diver. There
were three transect laid totally, and the distances
between each transect between 10 m. Diver swarm
along the sampling belt constant speed and recorded
a video perpendicularly to the seabed surface
underwater. The scleractinian species on each
section are photographed, to assist in the
identification of coral species, the more difficult to
identify the coral, to collect its skeleton from living
coral colonies and fixed in the alcohol for further
classification and identification in the laboratory.
Place square blocks of 0.5 m × 0.5 m equidistantly
on the spline (50 sample frames per sample strip, 1
m interval); Take vertical photographs of each
sample to further interpret the coral community
information. The video data is interpreted by
computer, and each sampling band was divided
evenly into 50 mark points. Measuring the points of
interest below the sample frame. The species and
number of scleractinian, rock, rubble, sand, sea
urchin and other were recorded, and the coverage,
distribution characteristics and health status of the
reef coral reefs are investigated by statistical
analysis. Each point was counted for the number of
points where the substrate was scleractinian coral
and the type of the corresponding coral. The ratio of
the coral cover point to the total number of points in
each sample was calculated, i.e. the coverage rate of
scleractinian corals; the occurrence of each coral
was counted. The scleractinian coral frequency is the
number of times this category appears in all sample
frames. Furthermore, the ratio of dead coral and
living coral cover was calculated with categories (1)
Healthy, if percent living to dead coral > 2:1, (2).
Fair/moderate, if between 2:1 and 1:2, (3) Unhealthy,
if live to dead coral < 1:2) (Reza and
Sancayaningsih 2015).
In this study, we refer to the morphological
classification of scleractinian species according to
the “Chinese Animal Records” volume 23 by Zhou
Renlin (Zhou R 2011) and “Corals of The World”
volume 1.2 and 3 by Veron (Veron 2000).
Species Diversity and Distribution of Scleractinian Coral at Daao Bay, Shenzhen
51
2.3 Data Analysis
Data was compiled and collated using MS.
Excel and SPSS 17.0. Analysis the diversity,
distribution characteristics and health status of
the s
cleractinian coral by Shannon-wiener index,
Simpson’s diversity index, Margarlef species
richness index, and Pielou index. We surveyed
the normal coral, dead and blanching coral
cover degree to assess the scleractinian corals
healthy condition at the same time. The
dominant s
cleractinian coral species in this area
was sorted by the important values of
scleractinian corals (importance value, IV). The
formulas were as follows:
H=
∑
𝑝
log
𝑝
(1)
D=1-
𝑝
(2)
M=(S-1)/log
2
N
(3)
J=𝐻log
𝑆
⁄
(4)
RA=The individuals of this scleractinian species / The
individuals of all scleractinian species
(5)
RF=Frequency of this scleractinian species /Total frequency
of all scleractinian species
(6)
RC=The coverage area of this species of scleractinian /Total coverage
area of all species of scleractinian
(7)
IV= RA + RF + RC
(8)
Where H is the scleractinian coral Shannon-
wiener Diversity index; p
i
is the proportion of
individuals belonging to species i in all individuals
(i.e., the ratio of species i coverage to total live
scleractinian coral coverage); D is the scleractinian
coral Simpson’s diversity index; M is the
scleractinian coral Margarlef species richness index;
N is the total scleractinian coral individual number
(i.e., total live scleractinian coral coverage); J is the
scleractinian coral Pielou index; S is total coral
species number; RA is the relative individuals
number of scleractinian coral; RF is relative
frequency of scleractinian corals; RC for relative
coverage rate; IV The is an important value for
scleractinian corals.
In this study, we classified the scleractinian
corals frequency into A to E 5 grades. Which the
scleractinian coral frequency between 1% and 20%
is zoned A level; Which the scleractinian coral
frequency between 20% and 40% is zoned B level;
Which the scleractinian coral frequency between 40%
and 60% is zoned C level; Which the scleractinian
coral frequency between 60% and 80% is zoned D
level; Which the scleractinian coral frequency
between 80% and 100% is zoned E level.
3 RESULTS AND DISCUSSIONS
3.1 Daao Bay Reef Coral Species
Composition
A total of 4 families, 8 genera, 13 species
scleractinian coral were recorded in this vessel,
based on coral morphology identification. The
Important Values (IV) sort results (table 1) indicates
that the main advantages of Daao Bay scleractinian
coral was Platygyra yaeyamaensis (IV =0.725), next
is porites lobate (IV =0.453). We divided
scleractinian coral into leaf-like corals, dendritic
corals and clumps coral according to the form. The
statistical results showed that there were 9 species
leaf-like coral, 2 species dendritic coral, 2 species
clumps coral in this area. The clumps coral take the
highest proportion, which is similar to the Daya Bay
nearly. Researches shows that clumps coral can
adapt the low temperature, suspended sediments,
environmental pollution etc. marine environment
changes better. As the latitude rises, the lower water
temperature, coral skeleton is also closer to the
clumps species (Riegl and Purkis 2009).
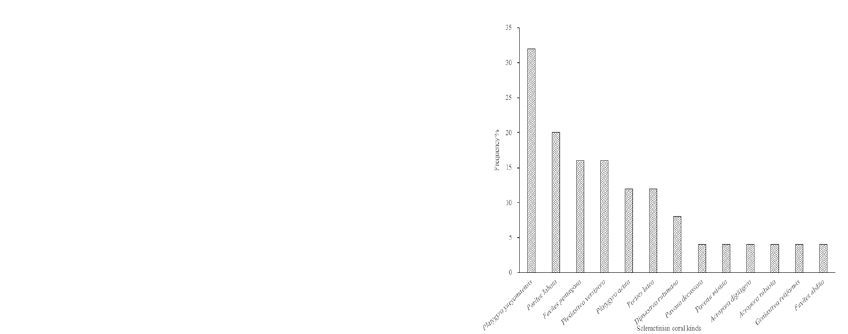
Figure 2: Frequency distribution of Scleractinian corals at
Daao Bay
IWEG 2018 - International Workshop on Environment and Geoscience
52
Table 1: Sorted importance value of scleractinian corals at Daao Bay
Rank Species P
i
RF RA RC IV
1 Platygyra yaeyamaensis 0.320 0.229 0.234 0.262 0.725
2 Porites lobata 0.200 0.143 0.106 0.203 0.453
3 Favites pentagona 0.160 0.114 0.149 0.059 0.322
4 Plesiastrea versipora 0.160 0.114 0.128 0.026 0.268
5 Platygyra acuta 0.120 0.086 0.106 0.043 0.235
6 Porites lutea 0.120 0.086 0.085 0.059 0.230
7 Dipsastrea rotumana 0.080 0.057 0.043 0.072 0.172
8 Pavona decussata 0.040 0.029 0.021 0.105 0.155
9 Pavona minuta 0.040 0.029 0.043 0.043 0.114
10 Acropora digitigera 0.040 0.029 0.021 0.059 0.109
11 Acropora robusta 0.040 0.029 0.021 0.056 0.106
12 Goniastrea retiformis 0.040 0.029 0.021 0.010 0.060
13 Favites abdita 0.040 0.029 0.021 0.003 0.053
3.2 Daao Bay Reef Coral Frequency
Distribution
The scleractinian coral distribution frequency
diagram (Figure 2) shows that 84.62% scleractinian
coral appears low frequency at Daao Bay sea area,
belongs to the A level; There were 15.38
% scleractinian coral species in B level; There is no
scleractinian coral species in C,D,E level. The five
frequency-level relationships was A>B>C=D=E.
The A level is far higher than the other levels. The
level C, D and E were 0 in this result. It indicate that
most of the species in this area is low frequency
distributing, even if the Platygyra yaeyamaensis,
which is frequency is only 32%. The 84.62% species
is fragmentary distribution around. The light,
transparency and other environment condition
determine the distribution characteristic. This area
was carrying out a reef restoration work. Reef
restoration is a novel ecological discipline that has
been receiving increasing attention over the past two
decades, though many of its theoretical and practical
aspects have yet to be elucidated (Rinkevich B
2015). It might be change the frequency distribution
and structure of community directly; the frequency
distribution would provide a simple parameter to get
the structure of community in the future. Recreation
of suitable condition for native communties’
development could alleviate ecological barriers to
reef regeneration (Horoszowski-Fridman and
Rinkevich 2017). The sparse planting model could
be more suitable in this area in the restoration works
compare to the intensive planting.
3.3 Reef Coral Diversity
This survey area scleractinian Coral Shannon-wiener
diversity index, Simpson’s diversity index and
Margarlef species richness index were 2.251 nit,
1.754 and 0.856, respectively. The Daao Bay coral
Shannon-wiener diversity index was higher than the
Daya bay (Shannon-wiener diversity index=1.754
nit). The scleractinian Coral Pielou index was 0.608
at Daao bay, higher than the Daya bay 0.305. This
survey had been recorded in total of 30 scleractinian
coral species, which is similar to that of coral reefs
in neighboring Daya bay waters (15 species), and
more than the Dongshan sea area in Fujian province
(5 species).
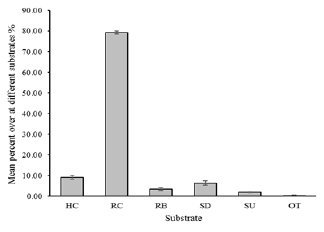
3.4 Bottom Coverage
The percent cover at a given station consists of
the mean of its three transects reef coral coverage of
the 2017 in the survey area is 9.12%, and the year of
Daao Bay Reef Coral coverage average 34.4% in
2007 (Jia C et al., 2008). The coral reef coverage in
Daao Bay has seriously declined recent 10 years. As
to this survey the highest coverage in this sea area
was rock (79.32%), and the sand, rubble, sea urchins,
other organisms were 6.38 %, 3.40 %, 1.78 %,
0.16 %, respectively. The scleractinian coral was
mainly distributed in the rock bottom and a small
amount of rock and gravel mixed bottom. Coral
bleaching considered an important index for
ecosystem health evaluating (Glynn 1993; Awak et
al., 2016). The survey results showed there were 0
deaths or bleaching corals were found in the survey
area. The ratio of living coral to dead coral cover
percent > 2:1, which indicating that the coral reefs in
Species Diversity and Distribution of Scleractinian Coral at Daao Bay, Shenzhen
53
the region are in good health condition recently. The
distribution of coral reef coverage shows that the
coverage of coral reefs in the Daao Bay was
heterogeneous, and the coverage interlaced high-low.
Sea urchins are important grazers and influence Reef
development in the eastern tropical Pacific
(Cabanillasterán et al., 2016). Researches show that,
sea urchins preferential resource appeared to be
benthic algal and turf, But if those were not
available, it feeds on other organisms, such as the
corals Pavona clavus, Pocillopora spp. and Porites
lobate (Reaka-Kudla et al., 1996). The sea urchin
coverage rate in this area was 1.78%. Because of the
complex dynamic balance of grazing animals
between sea urchins with coral reef
(Mcclanahan
and Shafir 1990, Nash et al., 2016). The further
tracking is needed to study the relationship between
sea urchins and coral reefs to better understand the
health of coral reef ecosystems in the region.
Figure 3. Coverage rates of different survey sample
substrates. (Note: a. HC: Hard Coral, RC: Rock, RB:
Rubble, SD: sand, SU: Sea urchin, OT: other. Error bars
correspond to standard deviation value.
)
4 CONCLUSION
The present survey results showed that the average
coverage of the scleractinian coral was 9.12% in the
Daao Bay, and the Shannon-wiener diversity index
was 2.25 nit, which declined seriously compared to
2007. Because of the incomplete sampling and the
drawback by morphological identification, there
might be some cryptic species that have not been
identified; the next stage will use molecular
biological means to classify the species diversity of
the coral (Wang et al., 2018;Daniel and Sergej, 2017;
Xin et al., 2017). Each zonation has its own pattern
in term of coverage and composition of corals life
form this pattern of coral distribution and abundance
with depth suggests this each coral species could
have an optimal depth (Hoogenboom et al., 2009).
For reasons such as transparency, light et al., the
vertical distribution of reef coral in this area was not
obvious (Yeung et al., 2014), and its main
distribution is in the depth of 3-10 m. The dominant
species of reef coral communities in this area was
mainly clumps of coral, followed by leaf-like corals.
Coral reef ecosystem is a marine ecosystem with
high productivity and diverse biodiversity, but also a
low resistance, very fragile ecosystem, as a
consequence of increasing human pressure, coastal
Ecosystems are facing a wide range of threats, such
as resource exploitation and habitat modification
(Rossi S 2013). Although there was no recent death
or blanching scleractinian coral have been found in
the survey area, but the coverage and diversity of
species were lower than historical data. The survey
sea area is close to human living quarters, coral reef
ecosystems are very easy to interfere with human
activities (Graham et al., 2017). Therefore, we
should strengthen the Daao Bay ecosystem
surveillance to protect the degenerative coastal coral
reef resources.
ACKNOWLEDGEMENTS
This research was jointly supported by Shenzhen
science and technology innovation project
(JCYJ20160331141759795).
REFERENCES
Amin AKMR. 2017 Diversity of Vibrios in the Coral Reef
Ecosystem of Ishigaki Island, Japan. 2017
Ateweberhan M and Mcclanahan TR 2016 Partitioning
scleractinian coral diversity across reef sites and
regions in the Western Indian Ocean. Ecosphere 7 1
Awak DSHL, Gaol JL, Subhan B, Madduppa HH and
Arafat D 2016 Coral Reef Ecosystem Monitoring
Using Remote Sensing Data: Case Study in Owi
Island, Biak, Papua. Procedia Environmental Sciences
33 600
Cabanillasterán N, Loorandrade P, Rodríguezbarreras R
and Cortés J 2016 Trophic ecology of sea urchins in
coral-rocky reef systems, Ecuador. Peerj 4 e1578
Cau A, Moccia D, Follesa MC, Alvito A, Canese S,
Angiolillo M, Cuccu D, Bo M and Cannas R 2017
Coral forests diversity in the outer shelf of the south
Sardinian continental margin. Deep Sea Research Part
I: Oceanographic Research Papers 122 60
Daniel HD and Sergej SJ 2017 Complete chloroplast
genome sequence of Castanopsis concinna (Fagaceae),
a threatened species from Hong Kong and South-
Eastern China. Mitochondrial DNA 28 65
IWEG 2018 - International Workshop on Environment and Geoscience
54
De Troch M, Melgoebarle JL, Angsincojimenez L,
Gheerardyn H and Vincx M 2008 Diversity and
habitat selectivity of harpacticoid copepods from sea
grass beds in Pujada Bay, the Philippines. Journal of
the Marine Biological Association of the UK 88 515
Eyal G, Wiedenmann J, Grinblat M, D'Angelo C,
Kramarskywinter E, Treibitz T, Benzvi O, Shaked Y,
Smith TB and Harii S 2015 Spectral Diversity and
Regulation of Coral Fluorescence in a Mesophotic
Reef Habitat in the Red Sea. Plos One 10 e128697
Facon M, Pinault M, Obura D, Pioch S, Pothin K, Bigot L,
Garnier R and Quod JP 2016 A comparative study of
the accuracy and effectiveness of Line and Point
Intercept Transect methods for coral reef monitoring
in the southwestern Indian Ocean islands. Ecological
Indicators 60 1045
Ferrigno F, Bianchi CN, Lasagna R, Morri C, Russo GF
and Sandulli R 2016 Corals in high diversity reefs
resist human impact. Ecological Indicators 70 106
Glynn PW 1993 Coral reef bleaching: ecological
perspectives. Coral Reefs 12 1
Graham NA, Mcclanahan TR, Macneil MA, Wilson SK,
Cinner JE, Huchery C and Holmes TH 2017 Human
Disruption of Coral Reef Trophic Structure. Curr Biol
27 231
Guevara CA and Breedy O 2004 Distribution, diversity,
and conservation of coral reefs and coral communities
in the largest marine protected area of Pacific Panama
(Coiba Island). Environ Conserv 31 111
Halik A and Verweij M 2017 Socio-cultural diversity and
public preferences for coral reef management options
in Indonesia. Ocean Coast Manage
Holden H and Ledrew E 2002 Measuring and modeling
water column effects on hyperspectral reflectance in a
coral reef environment. Remote Sens Environ 81 300
Hoogenboom MO, Connolly SR and Anthony KRN 2009
Effects of photoacclimation on the light niche of
corals: a process-based approach. Mar Biol 156 2493
Horoszowski-Fridman YB and Rinkevich B 2017
Restoration of the Animal Forests: Harnessing
Silviculture Biodiversity Concepts for Coral
Transplantation. 1313
Howlett S, Stafford R, Waller M, Antha S and Mason-
Parker C 2016 Linking Protection with the
Distribution of Grouper and Habitat Quality in
Seychelles. Journal of Marine Biology 1
Jia C, Huang M and Zhuang S. 2008 Coral resources
status and protection measures in Shenzhen. China
Fisheries 395 16
Luthfi OM and Anugrah PT 2017 Distribution of
Scleractinian coral as the main reef-building of coral
reef ecosystem in Karang Pakiman's Patch Reef,
Bawean Island. Jurnal Ilmu-Ilmu Perairan, Pesisir dan
Perikanan 5 9
Mcclanahan TR and Shafir SH 1990 Causes and
consequences of sea urchin abundance and diversity in
Kenyan coral reef lagoons. Oecologia 83 362
Moberg F and Folke C 1999 Ecological goods and
services of coral reef ecosystems. Ecol Econ 29 215
Muruganantham M, Ragavan P and Mohan PM 2017
diversity and distribution of living larger benthic
foraminifera from coral reef environments, south
andaman island, india. J Foramin Res 47 252
Nash KL, Graham NAJ, Jennings S, Wilson SK and
Bellwood DR 2016 Herbivore cross ‐ scale
redundancy supports response diversity and promotes
coral reef resilience. J Appl Ecol 53 646
Nyström M, Folke C and Moberg F 2000 Coral reef
disturbance and resilience in a human-dominated
environment. Trends Ecol Evol 15 413
Reaka-Kudla ML, Feingold JS and Glynn W 1996
Experimental studies of rapid bioerosion of coral reefs
in the Galápagos Islands. Coral Reefs 15 101
Reza A and Sancayaningsih RP 2015 Diversity
Distribution and Abundance of Scleractinian Coral in
District-based Marine Protected Area Olele, Bone
Bolango, Gorontalo-Indonesia. International
Conference on Biological Science, vol. 3. Yogyakarta,
Indonesia p.14.
Riegl BM and Purkis SJ 2009 Model of coral population
response to accelerated bleaching and mass mortality
in a changing climate. Ecol Model 220 192
Rinkevich B 2015 Climate Change and Active Reef
Restoration—Ways of Constructing the Reefs of
Tomorrow. Journal of Marine Science & Engineering
3 111
Roelfsema C, Kovacs E, Ortiz JC, Wolff NH, Callaghan D,
Wettle M, Ronan M, Hamylton SM, Mumby PJ and
Phinn S 2018 Coral reef habitat mapping: A
combination of object-based image analysis and
ecological modelling. Remote Sens Environ 208 27
Rossi S 2013 The destruction of the animal forests in the
oceans: Towards an over-simplification of the benthic
ecosystems. Ocean Coast Manage 84 77
Tun K and Wilkinson C 2004 The GCRMN - coordinating
coral reef monitoring efforts for effective management.
NAGA - The ICLARM Quarterly 40
Veron JEN 2000 Corals of the world: Australian Institute
of Marine Science
Wang X, Tian P, Niu W and Yu S 2018 The complete
mitochondrial genome of the Montipora peltiformi
(Scleractinia: Acroporidae). Mitochondrial DNA Part
B 3 99
Xin ZZ, Yu-Liu, Zhang DZ, Wang ZF, Zhang HB, Tang
BP, Zhou CL, Chai XY and Liu QN 2017
Mitochondrial genome of Helice tientsinensis
(Brachyura: Grapsoidea: Varunidae): Gene
rearrangements and higher-level phylogeny of the
Brachyura. Gene
Yeung CW, Cheang CC, Lee MW, Fung HL, Chow WK
and Ang Jr P 2014 Environmental variabilities and the
distribution of octocorals and black corals in Hong
Kong. Mar Pollut Bull 85 774
Zhou R 2011 Chinese Animal Records. Beijing: Beijing
Press
Species Diversity and Distribution of Scleractinian Coral at Daao Bay, Shenzhen
55
