
Isolation and Characterization of a Petroleum-Degrading
Pseudoalteromonas Haloplanktis Strain from the Digestive Tract of
Perinereis Aibuhitensis (Polychaete)
Bin Wang
1,*
, Baidong Zhang
2
and Yibing Zhou
1
1
Key Laboratory of Marine Bio-resources Restoration and Habitat Reparation in Liaoning Province, Dalian Ocean
University, Dalian, China;
2
Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences,
Qingdao, China.
Email: wangbin@dlou.edu.cn.
Keywords
: Polychaete, bioremediation, digestive tract, Pseudoalteromonas Haloplanktis
Abstract:
The application of bioremediation approaches employing hydrocarbon-utilizing microorganisms to remove
petroleum hydrocarbons from oil spills is an area of research that has gained extensive attention and has
been widely investigated. In the present study, attempts have been made to isolate and characterize
hydrocarbon-utilizing microorganisms immobilized in the digestive tract of Perinereis aibuhitensis. Isolate
SC11-3, a potent petroleum-degrading organism, from Perinereis aibuhitensis gut samples was identified as
Pseudoalteromonas sp. A detailed morphological, biochemical, and 16S rDNA sequence analysis revealed
that it was closely related to Pseudoalteromonas haloplanktis. The isolate SC11-3 was capable of
consuming about 40% diesel within 15 days from the medium containing 1 ml L
-1
of oil. Furthermore, it
was observed that the degrading efficiency of the isolate SC11-3 was significantly enhanced up to
approximately 90% when the medium was supplemented with 4 g L
-1
of glucose, indicating the possible
occurrence of co-metabolism during the process of petroleum degradation by the bacterium. Our study
reported an isolate of petroleum-degrading bacterium and its potential co-metabolism mechanism in oil
degradation processes, which will provide new insight into in situ bioremediation of multi-biological
systems.
1 INTRODUCTION
Marine contamination has become a major concern
as a result of increasing demand for imported
petroleum fuels and growing exploitation of marine
petroleum oil sources. Oil spill from transport
pipelines, storage tanks, and petroleum exploitation
could seriously pollute the marine environment and
disturb the surrounding ecosystem, mainly the
intertidal zone of the shoreline. Petroleum
hydrocarbon components are known to belong to the
family of neurotoxic and carcinogenic organic
contaminants (Nilanjana and Preethy 2011).
Bioremediation is considered as eco-friendly and
economic method to control petroleum pollution
owing to its advantages such as cost-effectiveness
and complete mineralization (Balba et al. 1998;
Vergeynst et al. 2018).
Recent studies have paid much attention to the
applications of bioremediation approaches
employing hydrocarbon-utilizing microorganisms to
remove petroleum hydrocarbon pollutants.
Microorganisms such as bacteria, fungi, yeasts, and
microalgae have the ability to mineralize petroleum
hydrocarbons (Atlas 1981; Leahy and Colwell 1990;
Ortega-González et al. 2015; Santos and Maranho
2018). However, the dilution of seeded
microorganisms or fertilizers is considered as one of
the major limitation for the application of
bioremediation processes (Radwan et al. 2002;
Panchal et al. 2018). As a result, there has been an
increasing interest in the use of marine sedimentary
invertebrates associated with petroleum-utilizing
bacteria in bioremediation as in situ multi-biological
24
Wang, B., Zhang, B. and Zhou, Y.
Isolation and Characterization of a Petroleum-Degrading Pseudoalteromonas Haloplanktis Strain from the Digestive Tract of Perinereis Aibuhitensis (Polychaete).
In Proceedings of the International Workshop on Environment and Geoscience (IWEG 2018), pages 24-31
ISBN: 978-989-758-342-1
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
approach for cleaning polluted marine environments.
There have been a few reports on organisms that can
degrade petroleum hydrocarbons or other high
molecular weight (HMW) polycyclic aromatic
hydrocarbons (PAHs), colonized in soil or other bio-
carriers, such as marine white-rot fungi and
autochthonous microflora (Radwan et al. 2002; Wen
et al. 2011).
During the past few decades the incidence and
threat of anthropogenic origins of petroleum
pollution has led to extensive research in isolation
and characterization of oil-degrading
microorganisms, particularly for the marine
environment. Most bacterial petroleum hydrocarbon
degraders have been isolated from heavily
contaminated coastal areas (Ridgway et al. 1990;
Mikesell et al. 1993, 1994; Watanabe et al. 1998;
Itagaki and Ishida, 1999; Kasai et al., 2001; Tazaki,
2003; Chaerun et al. 2004; Vergeynst et al. 2018;).
However, few studies have concentrated on
hydrocarbon-utilizing microorganisms immobilized
in the digestive tract of marine sedimentary
invertebrates, which exhibit high resistance to
pollution. Invertebrates participate actively in the
interactions that develop in sediment among
physical, chemical and biological processes, which
play significant roles in the delivery of ecosystem
services (Lavellea et al. 2006). Plants, invertebrates
and microorganisms have coevolved over several
hundred million years within soils. Invertebrates are
generally considered as key actors in the buffering
systems, which creates biogenic structures that may
act as incubators of microbial activities or microsites
for carbon and nutrient sequestration (Lavellea et al.
2006). For example, intestinal mucus plays a
significant role in the selection and stimulation of
microbial activities in the earthworm guts (Barois
and Lavelle 1986) and the effects of earthworm
cutaneous mucus on microbial selection have also
been demonstrated (Lavelle et al. 2005). Polychaetes
from the intertidal zone are known to accumulate
significant amounts of organic matter in addition to
biotransformation and elimination processes, which
make Polychaetes as candidate promoters and
indicators of oil-degrading mutualistic micro-
organisms.
Polychaetes present a wide geographical
distribution. Owing to their characteristics such as
short-distance migration and steady-state body
burden, polychaetes are known to accumulate
significant amounts of organic matter from the
environment and possess the ability to carry out
biotransformation and elimination processes (Chen
et al. 2012; Jørgensen et al. 2008). It is known that
the microbiota of the digestive tract has a crucial
impact on the host, and the interactions between
invertebrates and microorganisms are essential for
the bioremediation of marine sedimentary
environment because they affect organic matter
degradation and nutrient cycling (Byzov et al. 2007;
Knapp et al. 2009). Thus, the purpose of this study
was to isolate and identify potential petroleum-
degrading bacteria from the digestive tract of P.
aibuhitensis, and provide useful insight into in situ
bioremediation of multi-biological systems. In
addition, the capability of Pseudoalteromonas
haloplanktis to degrade diesel along with glucose as
a supplemented co-substrate of carbon source for
diesel degradation was also investigated. The results
of our study could be helpful in exploring the
possibility of cleaning polluted marine environments
in a more efficient way.
2 MATERIALS AND METHODS
2.1 Sample Collection and Isolation of
Microorganisms
Hydrocarbon-utilizing bacteria were isolated from
gut samples of P. aibuhitensis collected from the
shoreline of Panjin (Liaoning Province, China). Live
worms weighing approximately 5 g were transported
to the laboratory, and after being starved for 24 h,
the gut samples were removed using sterile forceps
and scissors on a super-clean bench. For the
isolation of hydrocarbon-utilizing bacteria, the
diluted homogenate of the gut samples was serially
diluted in sterile distilled water and daubed on solid
mineral medium (sterilized by autoclaving at 121℃,
15 psi for 15 min) supplemented with 1% (v/v)
sterile diesel as the sole carbon source.
Subsequently, the plates were incubated at 25°C for
5 days and screened for hydrocarbon-utilizing
bacterial colonies.
2.2 Identification and Characterization
of the Bacterial Isolates
The selected isolates were grown on 2216E agar
medium (peptone 5g, yeast extract 1g, powdered
agar 15g, Ferric phosphate 0.01g, seawater 1L,
pH7.6-7.8 and sterilized by autoclaving at 121℃, 15
psi for 15 min). The shape and colors of the colonies
Isolation and Characterization of a Petroleum-Degrading Pseudoalteromonas Haloplanktis Strain from the Digestive Tract of Perinereis
Aibuhitensis (Polychaete)
25
were screened out by observing bacterial form
properties of colony. In addition, the isolates were
also biochemically analyzed by conducting oxidase,
catalase, urease, V-P (Voges-Proskauer test), MR-
VP (methyl red test), nitrate reduction, oxidative
fermentation (OF), arginine dehydrolase, gelatin
hydrolysis, motility, glucose and citrate utilization,
TCBS (thiosulphate citrate bile salts) growth, and
O/129 drug susceptibility tests (Table 1). All the
identification tests were carried out according to
Bergey’s Manual of Systematic Bacteriology
(Williams and Wilkins 1986) and A Practical
Identification Manual of Bacteria from Fish and
Other Aquatic Animals (Nicky 2004).
2.3 Determination of Optimal Growth
Conditions
The optimal growth conditions with reference to pH,
temperature, and saline concentration were
determined. The strains were grown in 5 ml of
medium at varying pH values (5, 6, 7, 8, 9, and 10),
at different temperatures (5, 10, 15, 20, 25, 30, and
40°C), and with various NaCl concentrations (0%,
1%, 2%, 3%, 4%, and 5%), respectively. All
treatments were carried out in triplicate for 24 h with
shaking at 150 rpm. The optical densities of the
growing biomass under all the above-mentioned
conditions were assessed at 600 nm using an UV-
Vis spectrophotometer to determine the optimum
growth.
2.4 16s Rdna Sequencing, Alignment,
and Phylogeny
The isolates were purified using streaking method
before being subjected to sequencing (TaKaRa
Biotechnology (Dalian) Co., Ltd.). The full length of
the 16S rRNA genes (1450 bp) of the isolates was
amplified and sequenced using TaKaRa 16S rRNA
Bacterial Identification PCR Kit. The sequences
were analyzed for homology to other known
sequences matched with previously published
bacterial 16S rDNA sequences using the BLAST
program (Basic Local Alignment Search Tool).
Based on the scoring index, the most similar
sequences were aligned with the sequences of other
representative bacterial 16S rDNA regions (Woese
and Fox 1977), and a phylogenetic tree was
constructed using the neighbor-joining method with
Bootstrap of 1000.
2.5 Estimation of Bacterial Petroleum-
Degrading Efficiency
The bio-utilization of diesel was examined by using
fresh bacterial suspension (approximately 2×10
9
cfu/ml; 2% (v/v)) grown in 250-ml conical flasks
containing 100 ml of MMC medium (minimal
medium 1000ml, powdered agar 15g, Tween 80
1ml, pH7.2 and sterilized by autoclaving at 121℃,
15 psi for 15 min) supplemented with 0.1 ml of
sterile diesel. The flasks were incubated on a rotary
shaker at 150 rpm and 25°C for 3, 5, 7, 10, and 15
days, respectively and MMC medium without
inoculum was experimented as control group. All
treatments were carried out in triplicate, and the
residual oil of the samples was extracted at selected
time intervals by using petroleum ether
(transmittance: >90%; boiling range: 60–90°C). The
residual oil present in the solution was determined
by UV spectrophotometry at 221 nm (standard curve
was established by employing sterile diesel and
petroleum ether; R2=0.9995). The degradation rates
of the samples were estimated by using the MMC
medium without bacterial inoculum as control.
2.6 Effect of Glucose on Bacterial
Degrading Efficiency
The potentiality of microorganisms associated with
different concentrations of glucose as the
supplemented source of carbon for petroleum
degradation in sea water was determined
quantitatively. For this experiment, two different
inoculum concentrations were employed: about
0.5% (v/v) or 2% (v/v) (approximately 0.5 ml and
2.0 ml, respectively) of fresh bacterial suspension
(approximately 2×10
9
cfu ml-1) was added to each
flask containing 100 ml of the MMC medium with
0.1% (v/v) sterile diesel. Then, the flasks were
incubated on a rotary shaker at 150 rpm and 25°C
for 3 days. Three replicates were prepared for each
inoculum and glucose concentration (glucose
concentrations of 0.5, 1, 2, 4, 6, 8, 10, 16, 24, 32,
and 40 g L-1 for 0.5-ml inoculum, respectively;
glucose concentrations of 0.5, 1, 2, 4, 6, 8, 10, 16,
32, 48, 64, 80, and 96 g L-1 for 2.0-ml inoculum,
respectively). The number of oil-utilizing
microorganisms suspended in the water samples was
determined at the end of the incubation period by
employing spectrophotometry at 600 nm, and the
residual diesel in the MMC medium was recovered
by extraction with petroleum ether and
IWEG 2018 - International Workshop on Environment and Geoscience
26
quantitatively determined by using UV
spectrophotometry at 221 nm.
3 RESULTS
3.1 Isolation of Hydrocarbon-Utilizing
Strain
A total of three colonies were initially screened from
solid mineral medium supplemented with 1% (v/v)
sterile diesel as the sole carbon source. After
secondary screening, one of the potential strains,
isolate SC11-3, showing higher degree of oil
degradation rate was selected for further studies.
Morphological and biochemical analyses revealed
that the isolate SC11-3 was Gram-negative, rod-
shaped, and formed cream-yellowish colonies on
2216E agar medium. The isolate exhibited positive
results for catalase, urease, gelatin hydrolysis,
glucose utilization, motility, nitrate reduction, and
OF tests, and could grow at 4°C. Furthermore, the
results of 16S rRNA sequencing showed that the
isolate SC11-3 was closely related to P. haloplanktis
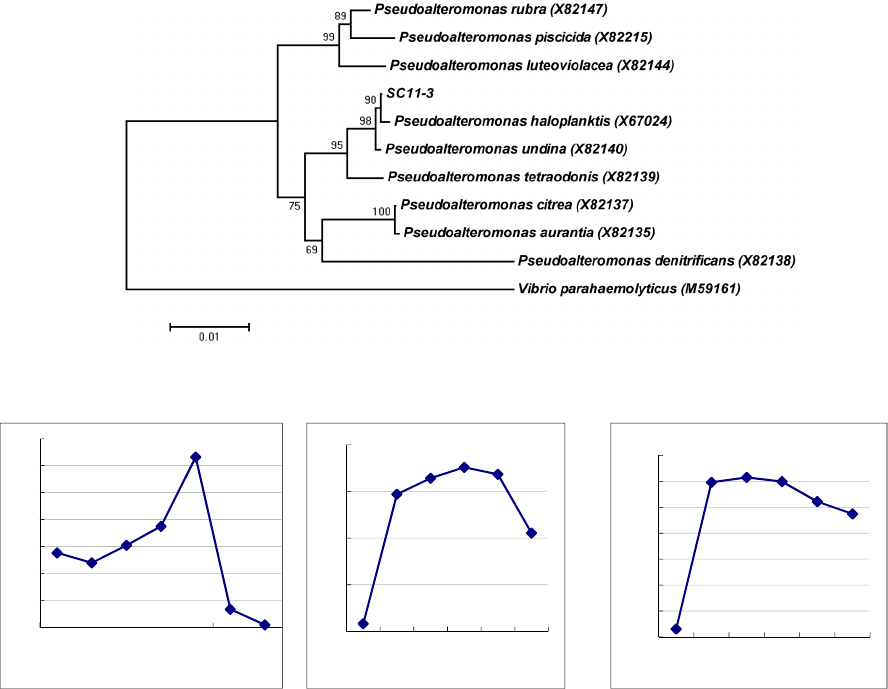
(Blast Max Identity 100%) (Figure 1).
Subsequently, the growth of the isolate SC11-3
was examined at different pH, temperatures, and
saline concentrations. After incubation for 24 h with
shaking at 150 rpm, the samples were removed to
assess the optical density of the growing biomass at
600 nm by using spectrophotometry. The results
revealed that the optimum growth temperature and
pH of the isolate SC11-3 was 25°C and 8,
respectively, and that the adaptive NaCl
concentration was in the range of 1–3%. In addition,
the isolate also exhibited a relatively stable tolerance
to low temperature (<15°C) and high NaCl
concentration (>3%) (Figure 2)
Figure 1: Phylogenetic analysis of 16S rRNA gene sequences of strain SC11-3 and related taxa. The scale bar
corresponds to 1% nucleotide sequence difference.
Figure 2: Effects of temperature pH and NaCl concentration on the growth of isolate SC11-3.
0
0,1
0,2
0,3
0,4
0,5
0,6
0,7
5 101520253040
OD/600nm
T/℃
Temperature
0
0,1
0,2
0,3
0,4
5678910
OD/600nm
pH
pH
0
0,05
0,1
0,15
0,2
0,25
0,3
0,35
012345
OD/600nm
NaCl%
Salinity
Isolation and Characterization of a Petroleum-Degrading Pseudoalteromonas Haloplanktis Strain from the Digestive Tract of Perinereis
Aibuhitensis (Polychaete)
27
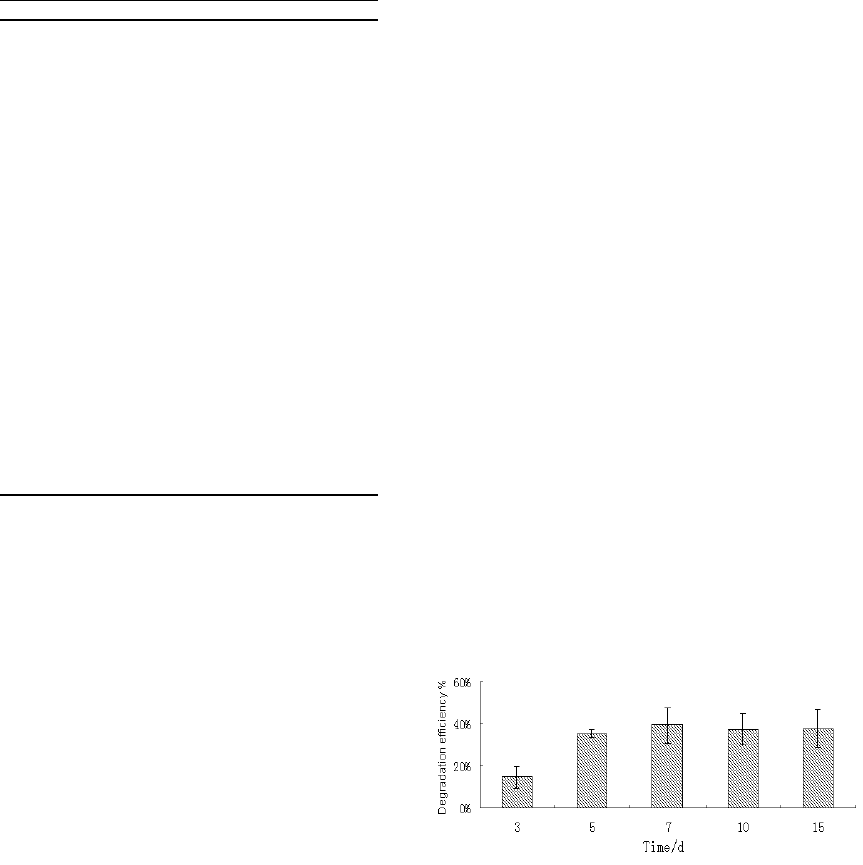
Table 1: Morphological and biochemical
characteristics of bacterial isolate SC11-3.
3.2 Bio-Utilization of Diesel
The diesel degrading capacity of the isolate SC11-3
was investigated by using 2% (v/v) inoculum at
different time intervals for up to 15 days. The
residual diesel concentration in the medium was
observed to decrease with the increasing cultivation
time for up to 15 days. To identify whether the
bacterial isolate could consume petroleum
hydrocarbons as the sole carbon and energy source,
diesel was used in this study. As shown in Figure 3,
the isolate SC11-3 was able to mineralize diesel as
the sole carbon and energy source for growth. The
highest bio-utilization capacity was observed on
Day 7, whereas no significant difference was found
among the degradation rates noted on Day 7
(39.47±8.37%), Day 10 (37.30±7.37%), and Day 15
(37.83±8.84%) (Figure 3). However, the diesel
degrading efficiency of the isolate was significantly
enhanced when glucose was added as an additional
carbon source. Furthermore, the change in the
biomass of the isolate SC11-3 was in good
agreement with the changes in the degradation rate
and glucose concentration. In general, in the
presence of glucose, the degradation rate of the
isolate was consistently higher than that of the
control (Figure 4). The diesel degradation efficiency
and growth of the isolate SC11-3 in the MMC
medium containing different concentrations of
glucose as a co-substrate of carbon source are shown
in Figure 4. Both the growth and degradation
efficiency of the isolate was restrained at low
glucose concentrations. In addition, there was a
positive correlation between the degradation
efficiency and growth of the isolate in the system
(0.5–2 g L-1); however, the correlation differed for
0.5% and 2% inoculum at glucose concentrations of
4–6 and 4–32 g L-1, respectively, as presented in
Figure 4(a) (b). Furthermore, for both 0.5% and 2%
inoculum, at a glucose concentration of 4 g/L, the
biomass decreased with the degradation efficiency
remaining relatively high up to approximately 90%
after the first appearance of the peaks of degradation
efficiency. Then, the growth gradually declined with
the increasing concentration of glucose, which may
be owing to the inhibitory effect of excessive
amount of glucose on the growth of the isolate.
Moreover, it was noted that 2.0% inoculum
presented relatively more potent tolerance to high
concentration of glucose.
Figure 3: Initial degradation efficiency of strain
SC11-3.
Characteristics P. haloplanktis SC11-3
Cell morphology rod
Colony colour cream yellowish
Colony diameter 1.5mm
Oxidase -
Catalase +
Urease +
V-P reaction -
MR-VP reaction -
Nitrate reduction +
OF +
Arginine dehydrolase -
Gelatin hydrolysis +
Motility +
Glucose utilization +
Citrate utilization -
TCBS growh -
O/129 susceptibility -
Gram staining -
Temperature (℃)
5+
15 +
25 +
30 +
40 -
Note : - negative; + positive.
IWEG 2018 - International Workshop on Environment and Geoscience
28
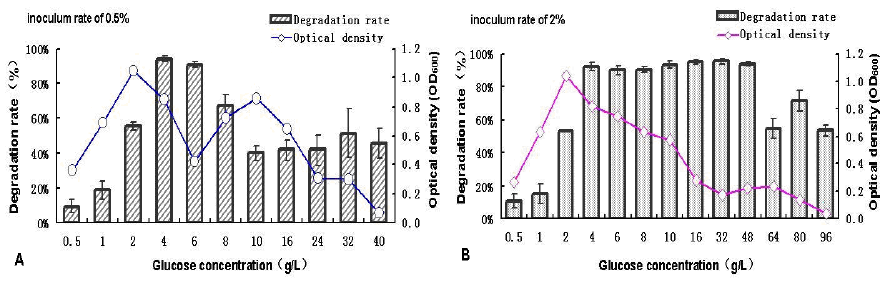
Figure 4. Effects of glucose concentrations on the degradation efficiency and biomass of strain SC11-3 at
inoculum rates of 0.5% and 2.0%.
4 DISCUSSIONS
In this study, we reported on a petroleum-degrading
strain, P. haloplanktis SC11-3, isolated from the gut
samples of P. aibuhitensis. The limit to higher
degradation rate was investigated based on the
concentration of glucose added as a supplementary
carbon source to the medium. The experimental
results indicated that the degree of growth and
degradation rate of the isolate SC11-3 varied with
the concentration of the glucose supplemented.
Furthermore, it was observed that the growth rates
of the isolate in the presence of glucose were
consistently higher than those of the control, and the
degradation rate was significantly improved at a
specific glucose concentration. However, with the
increase in the amount of glucose added to the
medium, the degradation rate declined, which may
be due to the inhibitory effect of excessive amount
of glucose on bacterial growth. Thus, the above-
mentioned results suggest the occurrence of co-
metabolism during the process of petroleum
degradation by the isolate SC11-3, because glucose
can either be consumed by the bacteria as a primary
carbon source through direct metabolism or used co-
metabolically when bacterial growth requires other
non-growth substrates.
Co-metabolism has the advantage of shortening
the lag phase in a biotreatment system (Volpe et al.
2009). Recent surveys have revealed that the most
important cause for the occurrence of co-metabolism
may be the increased activity or amount of microbial
biomass (Tittle et al. 1995). Furthermore, it has also
been reported that the main reason for the inability
of the microorganisms to efficiently degrade PAHs
is the lack of catabolic enzyme induction (Wen et al.
2011). Therefore, appropriate co-substrates such as
glucose may be useful for the bioremediation of
petroleum hydrocarbons because they can promote
efficient degradation. In addition, glucose has been
reported to stimulate the biodegradation of compost
(Jang et al. 2002), and co-metabolism has been
extensively applied to many areas of bioremediation
(Rentz et al. 2005; Xie et al. 2009). Nevertheless,
the applications of co-metabolism in petroleum
hydrocarbon degradation are scarcely reported.
A technical limitation in the bioremediation
process is the dilution of seeded microorganisms or
fertilizers (Radwan et al. 2002; Panchal et al. 2018).
As a result, there is an increasing interest in
investigating the use of marine sedimentary
invertebrates associated with petroleum-utilizing
bacteria in bioremediation, which shall provide new
efficient ways for cleaning polluted marine
environments. These multi-biological systems
provide suitable habitats for microorganisms, with
carbon source such as glucose, nitrogenous and
phosphorus compounds, and vitamins (Radwan and
Al-Muteirie 2001). Although the degrading
efficiency of P. haloplanktis in the present study
was observed to be significantly enhanced, more
studies will be further performed by our group on in
situ bioremediation process on the laboratory scale,
such as the degrading efficiency under anoxic
conditions as well as association with Perinereis
aibuhitensis and glucose. The petroleum-utilizing
bacterium identified in the present study could be
used in bioremediation as a potential candidate for
Isolation and Characterization of a Petroleum-Degrading Pseudoalteromonas Haloplanktis Strain from the Digestive Tract of Perinereis
Aibuhitensis (Polychaete)
29
being artificially immobilized in the digestive tract
of worms, which will provide a useful insight into in
situ bioremediation of multi-biological systems.
5 CONCLUSIONS
In the present study, Isolate SC11-3 was identified
and characterized as a potential hydrocarbon-
utilizing microorganisms immobilized in the
digestive tract of Perinereis aibuhitensis, a marine
sedimentary invertebrate with high resistance to
pollution. Our study reported the effect of co-
metabolism on the activation of petroleum-
degrading potential of Isolate SC11-3. Although the
mechanism precisely responsible for such effects are
generally not known, the current research is starting
to unravel the mechanisms for such oil degradation
processes. Thus, the findings of bacterial isolate of P.
haloplanktis here could be used for more detailed
future investigations on the oil-degrading genes and
environmental factors influencing the
bioremediation mechanism. The results of our
present study could be helpful in exploring the
possibility of cleaning polluted marine environments
in a more efficient way and provide new insights
into in situ bioremediation of multi-biological
systems.
REFERENCES
Atlas R M 1981 Microbial hydrocarbon of petroleum
hydrocarbon: an environmental perspective. Microbiol
Rev 45 180–209
Balba M T, Al A N, Al D R and Heitzer A 1998
Bioremediation of oil-contaminated soil:
microbiological methods for feasibility assessment
and field evaluation. Journal of Microbiological
Methods 32 155–164
Barois I and Lavelle P 1986 Changes in respiration rate
and some physicochemical properties of a tropical soil
during transit through Pontoscolex corethrurus
(Glossoscolecidae, Oligochaeta). Soil Biology &
Biochemistry 18 (5) 539-541
Byzov B A, Khomyakov N V, Kharin S A and Kurakov A
V 2007 Fate of soil bacteria and fungi in the gut of
earthworms. European Journal of Soil Biology 43
149–156.
Chaerun S K, Tazaki K, Asada R and Kogure K 2004
Bioremediation of coastal areas 5 years after the
Nakhodka oil spill in the Sea of Japan: isolation and
characterization of hydrocarbon-degrading bacteria.
Environment International 30 911–922
Chen X, Zhou Y, Yang D, Zhao H, Wang L and Yuan X
2012 CYP4 mRNA expression in marine polychaete
Perinereis aibuhitensis in response to petroleum
hydrocarbon and deltamethrin. Marine Pollution
Bulletin 64 1782–1788
Jang J C, Shin P K, Yoon J S, Lee I M, Lee H S and Kim
M N 2002 Glucose effect on the biodegradation of
plastics by compost from food garbage. Polymer
Degradation and Stability 76 155–159
Jørgensen A, Giessing A M B, Rasmussen L J and
Andersen O 2008 Biotransformation of polycyclic
aromatic hydrocarbons in marine polychaetes. Marine
Environmental Research 65 171–186
Knapp B A, Podmirseg S M, Seeber J, Meyer E and Insam
H 2009 Diet-related composition of the gut microbiota
of Lumbricus rubellus as revealed by a molecular
fingerprinting technique and cloning. Soil Biology &
Biochemistry. 41 2299–2307
Lavelle P, Rouland C, Diouf M, Binet F and Kersanté A
2005 Regulation of microbial activities by roots and
soil invertebrates. Journal of Bacteriology 179 (1)
7856-7864
Lavellea P, Aubert M and Barot S, et al. 2006 Soil
invertebrates and ecosystem services. European
Journal of Soil Biology
42 (11) S3–S15
Leahy J G and Colwell R R 1990 Microbial degradation of
hydrocarbons in the environment. Microbiol Rev 54
305–315
Mikesell M D, Kukor J J and Olsen R H 1993/1994
Metabolic diversity of aromatic hydrocarbon-
degrading bacteria from a petroleum-contaminated
aquifer. Biodegradation 4 249–259
Nicky B B 2004 Bacteria from Fish and Other Aquatic
Animals: A Practical Identification Manual
Nilanjana D and Preethy C 2011 Microbial Degradation of
Petroleum Hydrocarbon Contaminants: An Overview.
Biotechnology Research International 1 941810
Ortega-González D K, Martínez-González G and Flores C
M, et al. 2015 Amycolatopsis sp. Poz14 isolated from
oil-contaminated soil degrades polycyclic aromatic
hydrocarbons. International Biodeterioration &
Biodegradation 99165-173
Panchal A, Swientoniewski L T and Omarova M, et al.
2018 Bacterial proliferation on clay nanotube
Pickering emulsions for oil spill bioremediation.
Colloids & Surfaces B Biointerfaces 164 27-33
Radwan S S and Al-Muteirie A S 2001 Vitamin
requirements of hydrocarbon-utilizing soil bacteria.
Microbiological Research 155 301–307
Radwan S S, Al-Hasan R H, Salamah S and Al-Dabbous S
2002 Bioremediation of oily sea water by bacteria
immobilized in biofilms coating macroalgae.
International Biodeterioration & Biodegradation 50
55–59
Rentz J A, Alvarez P J J and Schnoor J L 2005
Benzo[a]pyrene co-metabolism in the presence of
plant root extracts and exudates: implications for
phytoremediation. Environmental Pollution 136 477–
IWEG 2018 - International Workshop on Environment and Geoscience
30
484
Ridgway H F, Safarik J, Phipps D, Carl P and Clark D
1990 Identification and catabolic activity of well-
derived gasoline-degrading bacteria from a
contaminated aquifer. Applied and Environmental
Microbiology 56 3565–3575
Santos J J D and Maranho L T 2018 Rhizospheric
microorganisms as a solution for the recovery of soils
contaminated by petroleum: A review. Journal of
Environmental Management 210 104-113
Tittle P C, Liu Y T, Strand S E and Stensel H D 1995 Use
of alternative growth substrates to enhance PAH
degradation. In: R. E. Hinchee (eds) Bioremediation of
Recalcitrant Organics 1–7
Vergeynst L, Wegeberg S and Aamand J, et al. 2018
Biodegradation of marine oil spills in the Arctic with a
Greenland perspective. Science of the Total
Environment 626 1243-1258
Volpe A, Del M G, Rossetti S, Tandoi V and Lopez A
2009 Enhanced bioremediation of methyl tert-butyl
ether (MTBE) by microbial consortia obtained from
contaminated aquifer material. Chemosphere 75 149–
155
Watanabe K, Teramoto M, Futamata H and Harayama S
1998 Molecular detection, isolation, and physiological
characterization of functionally dominant phenol-
degrading bacteria in activated sludge. Applied and
Environmental Microbiology 64 4396–4402
Wen J W, Gao D W, Zhang B and Liang H 2011 Co-
metabolic degradation of pyrene by indigenous white-
rot fungus Pseudotrametes gibbosa from the northeast
China. International Biodeterioration &
Biodegradation 65 600–604
Williams, Wilkins 1986 Bergey’s Manual of Systematic
Bacteriology. ISBN 0-683-04108-8
Woese C R and Fox G E 1977 Phylogenetic structure of
the prokaryotic domain: the primary kingdoms.
Proceedings of the National Academy of Sciences of
the United States of America 74 (11) 5088–5090
Xie S, Liu J X, Li L and Qiao C L 2009 Biodegradation of
malathion by Acinetobacter johnsonii MA19 and
optimization of cometabolism substrates. Journal of
Environmental Sciences 21 76–82
Isolation and Characterization of a Petroleum-Degrading Pseudoalteromonas Haloplanktis Strain from the Digestive Tract of Perinereis
Aibuhitensis (Polychaete)
31
