
Histological Change of Pancreatic Islands Following Administration
of Saurauia vulcani Korth Leaves Extract in Alloxan-induced
Diabetic Mice
Salomo Hutahaean
1
, S. Ilyas
1
, S. Rahayu
1
1
Department of Biology, Faculty of Mathematics and Natural Sciences,
Universitas Sumatera Utara, Jl. Bioteknologi No. 1, Kampus USU, Medan, Indonesia 20155.
Keywords: Saurauia vulcani, antiglycemic, Alloxan-induced diabetes, blood glucose level
Abstract: The importance of plant-based ingredients in traditional diabetes therapy has been well known. In North
Sumatra, one of the local species used was Saurauia vulcani Korth. plant (pirdot). The study was intended to
investigate the effect of S. vulcani leaves extract on the histology of pancreatic island in alloxan-induced
diabetic mice. Thirty male mice were divided into three groups of ten mice, namely: Normal Mice (NM)
group, Non-Treated Diabetic Mice (NTDM) group, and Treated Diabetic Mice (TDM) group which is given
200 mg/kg bw leaves extract of S. vulcani daily for 21 days by oral gavage. Diabetes was induced in mice by
alloxan injection. Blood sugar levels (BGL) and body weight were measured on day 0 (baseline, time of
alloxan injection), 72 hours, and on 7, 14, and 21 days after diabetes induction. On the 21st day, the mice
were sacrificed and the pancreatic organ was isolated and 8 micron tissue sections were made and stained
with Haematoxylin and Eosin. The results showed that BGL in NTDM group increases until the end of the
experiment, while in TDM it increases first but then decreases to the level that similar to NM control group
which is relatively stable around 100 mg/dl during the study. In histological examination, irregular island
forms, loose cell population, and cells with pyknotic nucleus were found, as an indication of a damage to the
pancreatic island. These changes were not found in NM groups and TDM groups. Our result indicated that S.
vulcani extract promotes cell regeneration in pancreatic islands. The results indicated that S. vulcani leaves
extract promotes cell regeneration in pancreatic islands.
1 INTRODUCTION
Diabetes (Diabetes mellitus, DM) is a metabolic
disease with a high prevalence rate. Globally, the
number of people with diabetes in the year 2000 was
171 million (2.3%), this figure is predicted to increase
to 368 million (4.4%) in 2030 (Wild, 2004). The main
characteristic of diabetes is high blood glucose levels
(BGL). High BGL can be caused by impaired insulin
secretion in the pancreas, impaired insulin action in
pheripheral tissue, or a combination of both. The
continued effects of chronic diabetes can cause
damage, dysfunction, and failure in various organs
(Saikh, 2016). The treatment of diabetes includes
injecting insulin, using drugs that can increase
pancreatic secretion activity, and drugs that can
increase tissue response to insulin. Diabetes
medications such as sulfonylurea analogues, alpha-
glucosidase inhibitors, and biguanides have side
effects, such as lowering BGL to a very low levels
(hypoglycemia effect), hepatotoxicity, lactic acidosis,
and diarrhea. (Fowler, 2007). Therefore, there is a
need to look for new agents that meet the
requirements as an ideal antidiabetic compound. The
candidates compound which is ideal as an anti-
diabetic drug is an agent that can reduce blood sugar
levels and simultaneously increasing the pancreatic
beta cell population. One strategy that can be done is
to test the antihyperglycemic activity and the effect
on pancreatic organ of plants extract that have
traditionally been used by people as a drug for
diabetes.
In one province in Indonesia (North Sumatra
province), the leaves of pirdot plants (Saurauia
vulcani Korth.) have long been used as a drug for
diabetes (Situmorang., 2015). This article reports the
effect of S. vulcani leaf extract on the histology of
pancreatic island in alloxan-induced diabetic mice.
Hutahaean, S., Ilyas, S. and Rahayu, S.
Histological Change of Pancreatic Islands Following Administration of Saurauia vulcani Korth Leaves Extract in Alloxan-induced Diabetic Mice.
DOI: 10.5220/0010104010951098
In Proceedings of the International Conference of Science, Technology, Engineering, Environmental and Ramification Researches (ICOSTEERR 2018) - Research in Industry 4.0, pages
1095-1098
ISBN: 978-989-758-449-7
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
1095

2 MATERIALS AND METHODS
2.1 Preparation of Plant Extract
The leaves of S. vulcani were obtained from the
forest of Sintongmarnipi, Toba Samosir, North
Sumatra Province, Indonesia. The leaves are picked,
cleaned, and separated from the petiolus, then dried
in open air for about one week. The dried leaves are
powdered into fine flour using an electric blender.
The extraction process was carried out by maceration
method using ethanol as a solvent. The maceration
results obtained are then filtered and the solvent was
evaporated using a rotary evaporator at 40 ° C
(Sitorus, 2015).
2.2 Experimental Animals
The experimental animals used were mice (Mus
musculus L.), male, healthy, aged ± 3 months,
weighing 25-30 grams. Animal experiments were
obtained from and maintained in animal cages
Department of Biology FMIPA USU Medan. Mice
are kept in a special cage for experimentation, given
pellet feed and drinking water (tap water) on an ad
libitum basis. The animals were adjust to a new
environment by kept them in the cage for two weeks
before experimentation.
Induction of diabetes in mice was carried out by
giving a single injection of alloxan (100 mg/kg body
weight) intraperitoneally. Mice were fasted 12 hours
before the injection. Selection of mice was done 72
hours after the alloxan injection. Only mice that have
a blood glucose level (BGD) >200 mg/dl are included
in the experiment.
The experiment was designed in completely
randomly designed (CRD).Thirty diabetic mice (BGL
>200) were randomly assigned to three treatment
groups of 10 mice each. The treatment groups were:
normal mice as control (NM groups), Non-Treated
Diabetic Mice (NTDM groups), and Treated Diabetic
Mice (TDM groups) that received S. vulcani leaves
extract 200 mg/kg body weight. The treatment was
given daily by oral gavage for 21 days.
2.3 Blood Glucose Level and Body
Weight
Blood glucose level was measured on blood removed
from the tail of the mouse. BGL determination is
performed with a glucometer (EasyTouch). BGL
measurements were carried out on day 0 (baseline) at
the time of alloxan injection, then 72 hours after the
injection, and then on the 7th, 14th, and 21st days.
About 5 mm the tip of the mouse's tail was cut with
scissors, then the blood was left to drip to a special
test strip for glucose and after about 10 seconds the
BGL number will appear on the glucometer screen.
The body weight was weighed on the same day
as the BGL determination day by using an electronic
scale.
2.4 Tissue Preparation
Animals were sacrificed at the end of the experiment.
Pancreatic organs were isolated and fixed in
formaldehyde. Tissue section was prepared by the
paraffin method. Eight μm thick tissue sections were
stained with HE and used for pancreatic island
observation.
2.5 Statistic Analysis
Parametric data were analyzed with ANOVA, the
Duncan 'test post hoc was apllied for all ANOVA
significant result. The differences between means
were considered significant at p<0.05.
3 RESULT AND DISCUSSION
Results from ANOVA showed that at the time of
alloxan injection (baseline, day 0) blood glucose level
(BGL) between treatment groups was not
significantly different (p>0.05). In the group of
normal mice (NM) the BGL baseline was around 100
mg/dl. This level was stable in NM group until the
end of the experiment.
Significant differences in BGL between
treatments began to be seen in observations 72 hours
after alloxan injection, whereas mice injected with
alloxan increased their blood glucose levels up to>
200. In figure 1, BGL in the NTDM group continued
to increase to> 250 mg/dl. The increasing level of
BGL in NTDM group was believed due to the
development of diabetic condition. In contrary, the
BGL in the TDM group was dropped after the animal
received S. vulcani leaves extract. The BGL
decreases was detected on day-7, but the significant
different to NTDM group (p<0.05) started on day-14.
The level continues to decrease until it reaches a level
that is not significantly different from the control
(NM group) at the end of the study (p>0.05).
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
1096
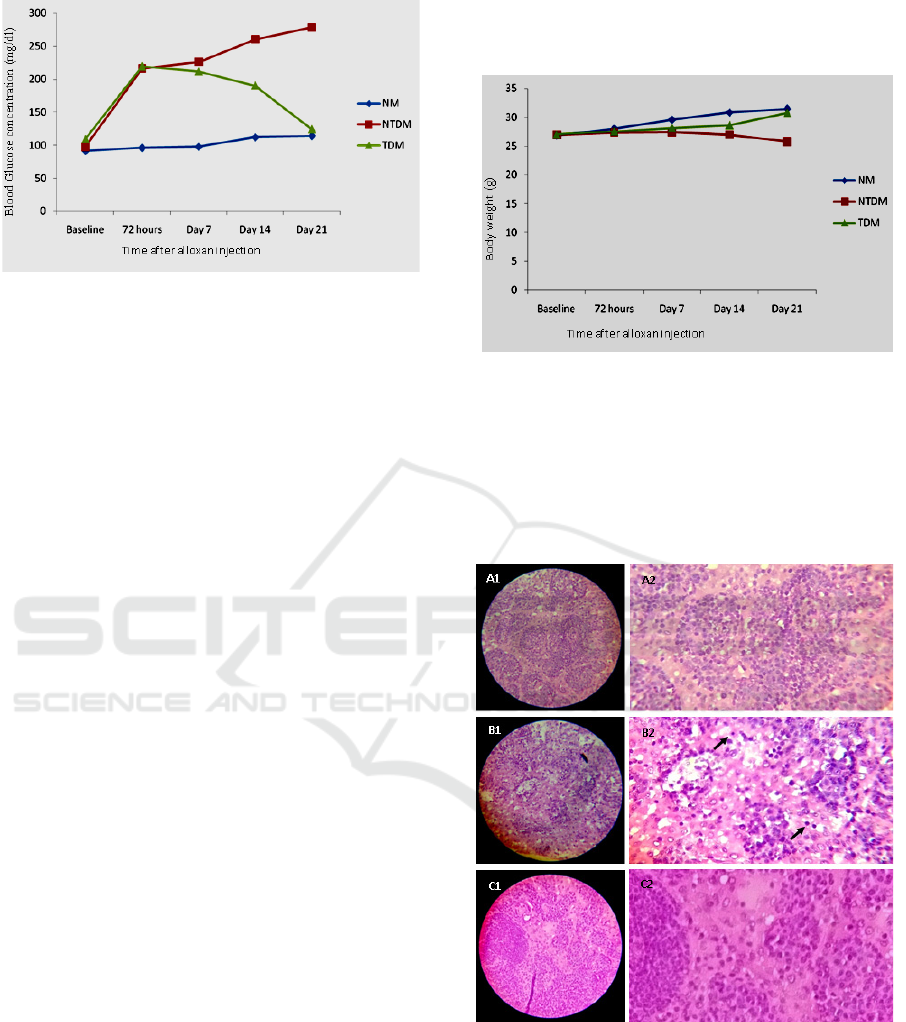
Figure 1. The change in mean of blood glucose level in
normal mice group (NM) group, Non-Treated Diabetes
Mice (NTDM), and Treated Diabetes Mice (TDM) at
different time of observation. The TDM mice received 200
mg/kg body weight leaves extract (Saurauia vulcani Korth).
The effect of treatments on body weight is shown
in figure 2. There is no significant difference in body
weight between treatments both at baseline and at 72
hours after alloxan injection, but on day 7 the body
weight in NTDM and TDM groups is lower than body
weight in control (NM) (p<0.05). These conditions,
especially in the NTDM group, continued until the
end of the study, while in the TDM group the body
weight improved to levels that were not different
significantly from controls (NM).
These results indicated that alloxan induces
diabetes in mice characterized by an increase in BGL
and then followed by weight loss. The results
obtained was agree with the previous studies
(Ewenighi,., 2015; Hutahaean., 2018). Uncontrolled
diabetes causes an increase in glycogenolysis,
lipolysis, and gluconeogenesis. Biochemical
activities through these three pathways are basically
the use of a new energy source for the body to
compensate for the low glucose entering the cell. The
use of energy sources from body fat and protein in
these processes is believed to be the pathway for the
mechanism of weight loss in diabetes.
The mechanism of diabetes induction by alloxan
is by damaging the pancreatic beta cells through the
resulting free radical effects. Alloxan is a glucose
analog which is specifically accumulates in the
pancreatic beta cells. By intracellular thiol activity,
especially glutathione, alloxan produces reactive
oxygen species (ROS) in a cyclic redox reaction with
its product, dialuric acid. Dialuric acid autoxidation
produces superoxide radicals, hydrogen peroxide
and, hydroxyl radicals. Hydroxyl radicals are
responsible for the death of beta cells that have very
low antioxidant defense abilities. As a thiol reagent,
alloxan also selectively inhibits glucose-induced
insulin secretion through its ability to inhibit glucose
sensor in beta cells (Szkudelski, 2001; Lenzen, 2008).
Histological observation showed the damage of
pancreatic cells in the NTDM group (figure 3).
Figure 3. The change of pancreatic island feature after
Saurauia vulcani leaves extract treatment in alloxan-
induced diabetic mice. A1 and A2: normal mice (NM); B1
and B2: Non-Treated Diabetes Mice (NTDM); C1 and C2:
Treated Diabetes Mice (TDM). The TDM mice received
200 mg/kg body weight leaves extract (Saurauia vulcani
Korth). Staining HE; 400 X.
Figure 2. The change in mean body weight in normal mice
group (NM) group, Non-Treated Diabetes Mice (NTDM),
and Treated Diabetes Mice (TDM) at different time o
f
observation. The TDM mice received 200 mg/kg bod
y
weight leaves extract (Saurauia vulcani Korth).
Histological Change of Pancreatic Islands Following Administration of Saurauia vulcani Korth Leaves Extract in Alloxan-induced Diabetic
Mice
1097

The damages were cells with a pyknotic nucleus,
cell populations reduction which was characteristics
of a more loose group of cells in the island (figure 3,
B1 and B2). In NTDM group, island cell population
reduced, the boundaries of the islands appear
irregular, and cells damage of pyknotic type found
(black arrow). Those were not found in control (NM)
and in mice treated with Saurauia vulcani leaves
extract (TDM group).
In addition, the forms of islands also appear
irregular compared to the control group (NM). In the
TDM group, there were indications of improvement
in the morphological structure of the pancreas which
was characterized by the denser features of island
cells, no more pyknotic cells, and more regular island
boundaries. The morphological changes obtained was
supporting the findings of BGL repair due to the
treatment of S. vulcani leaves extract.
ACKNOWLEDGEMENTS
This research was funded by the Directorate of
Research and Community Service of the Directorate
General of Research Strengthening and Development
of the Ministry of Research, Technology and Higher
Education, in accordance with the Research Funding
Agreement and Community Service in Fiscal Year
2018.
REFERENCES
Ewenighi C, U Dimkpa, J Onyeanusi, L Onoh, G
Onoh, U Ezeugwu. 2015. Estimation of glucose
level and body weight in Alloxan Induced
Diabetic Rat treated with Aqueous extract of
Garcinia Kola Seed. Ulutas Med J. 1(2): 26-30.
doi: 10.5455/umj.20150507042420.
Fowler, M. J. 2007. Diabetes treatment, Part 2: Oral
agents for glycemic management. Clin. Diabetes
25, 131–134.
Hutahaean, S., Tanjung, M., Sari, D. P. & Ningsih, V.
E. 2018. Antihyperglycemic and
antihyperlipidemic effects of pirdot (Saurauia
vulcani Korth.) leaves extract in mice. IOP
Conference Series: Earth and Environmental
Science, Vol. 130, Conference 1.
Lenzen S. 2008. The mechanisms of alloxan- and
streptozotocin-induced diabetes. Diabetologia.
Feb;51(2):216-26.
Shaikh, H., Shrivastava, V. K. & Amir, M. 2016.
Diabetes Mellitus and Impairment of Male
Reproductive Function: Role of Hypothalamus
Pituitary Testicular Axis and Reactive Oxygen
Species. 8, 41–50.
Sitorus, P. 2015. Characterization Simplisia and
Ethanolic Extract of Pirdot ( Saurauia Vulcani ,
Korth ) Leaves and Study of Antidiabetic Effect
in Alloxan Induced Diabetic Mice. International
Journal of ChemTech Research 8, 789–794.
Situmorang, R., Harianja, A., & Silalahi, J. 2015.
Karo’s Local Wisdom: The Use of Woody Plants
for Traditional Diabetic Medicines. Indonesian
Journal of Forestry Research, 2(2), 121-130.
doi:http://dx.doi.org/10.20886/ijfr.2015.2.2.121-
130.
Szkudelski, T. 2001. The mechanism of alloxan and
streptozotocin action in B cells of the rat pancreas.
Physiol. Res. 50, 537–546.
Wild, S. 2004. Global Prevalence of Diabetes
Estimates for the year 2000 and projections for
2030. World Health 27, 1047–1053.
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
1098
