
The Effect of Chives (Allium schoenoprasum L.) Leaves Ethyl
Acetate and Hexane Fractions on Human Calcium Kidney Stones
Solubility by In Vitro Method
Siti Morin Sinaga
1
, Sudarmi
1
and Iksen
1
1
Department of Pharmaceutical Chemistry, Universitas Sumatera Utara, Jl. Tridharma Kampus USU ,Medan, Indonesia
Keywords: Chives, Kidney Stones, Calcium, Fraction, Solubility.
Abstract: The aim of this research was to find out whether the ethyl acetate and hexane fractions had an effect to
dissolve the level of calcium in human kidney stones.The solubility activity of chives leaves ethyl acetate
and hexane fraction were carried out by using the in vitro method which was to test the solubility of the
calcium components of human kidney stones in various concentrations (1%, 2.5% and 5%). The fractions
were added by human kidney stones and incubated on 37
o
C for four hours. The ammount of calcium
solubility was measured by using atomic absorption spectrophotometer at 422.7 nm. The results showed that
ethyl acetate fraction had a dissolving effect of calcium on the human kidney stone which are respectively
18.98 µg/mL, 32.84 µg/mL and 26.22 µg/mL better than hexane fraction which are respectively 2.02 µg/mL,
2.45 µg/mL and 3.03 µg/mL In these results, the concentration of 2.5% of ethyl acetate fraction showed the
highest dissolving level which is 91.99%. Based on the research, it can be concluded that ethyl acetate
fraction of chives leaves is a new potential for herbal treatment of kidney stones.
1 INTRODUCTION
Kidney stones are one of the most common diseases
in the kidneys. Generally, kidney stones occur
because the body lacks fluids resulting in blockages
in the channels from the kidneys to the bladder. The
stones in the kidney are mainly formed from
chemicals that are usually found in urine such as
calcium, uric acid, phosphate, cystine and others
chemicals compound. Different types of treatments
are applied for kidney diseases such as medicine,
changing of life style, treatment by surgery etc.
(AUA, 2014; Mugni, 2013).
According to Al-Snafi (2013) and Iksen (2015),
chives are believed to be a multifunctional medicine
for various diseases. Chives contain various
phytochemical compounds including alkaloids,
flavonoids, glycosides, steroids, tannins and various
minerals such as potassium, calcium, magnesium
and sodium. Previous research showed that infuse
water of chives has a calcium oxalate solubility [5].
However, until now there is no scientific proof of
human calcium kidney stone of ethyl acetate and
hexane fractions of chives leaves. The purpose of
this study is to study the effect of chives leaves ethyl
acetate and hexane fractions on human calcium
kidney stones solubility.
2 MATERIALS AND METHODS
2.1 Materials
Distilled water, human calcium kidney stones, fresh
chives leaves, nitric acid 65% (Merck), ethanol
solvent (Merck), ethyl acetate solvent (Merck),
hexane solvent (Merck) and standard solution of
calcium 1000 ppm.
2.2 Plant Material Preparation
Fresh chives leaves was collected from local area of
Simalungun distric (North Sumatera, Indonesia) and
authenticated by Herbarium Medanese (MEDA)
Universitas Sumatera Utara. Voucher specimen was
collected and deposited in Pharmacognosy
Laboratory, Faculty of Pharmacy, Universitas
Sumatera Utara.
914
Sinaga, S., Sudarmi, . and Iksen, .
The Effect of Chives (Allium schoenoprasum L.) Leaves Ethyl Acetate and Hexane Fractions on Human Calcium Kidney Stones Solubility by In Vitro Method.
DOI: 10.5220/0010101109140916
In Proceedings of the International Conference of Science, Technology, Engineering, Environmental and Ramification Researches (ICOSTEERR 2018) - Research in Industry 4.0, pages
914-916
ISBN: 978-989-758-449-7
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
2.3 Preparation of Fraction
Extraction was done by maceration method using
ethanol solvent. 200 g of powdered chives leaves
were marcerated in 1 L ethanol solvent 24 hours,
then filtered, do it continuously until the filtrate
obtained is clear and colorless. 10 g concentrated
ethanolic extract, then fractioned using ethyl acetate
and hexane solvents to get the ethyl acetate and
hexane fractions (Ditjen POM, 1995; Depkes RI
1995).
2.4 Experimental Design
In this research, samples were divided into 6 groups,
which were namely EA1 (1% ethyl acetate
fraction), EA2 (2.5% ethyl acetate fraction), EA3
(5% ethyl acetate fraction), H1 (1% hexane
fraction), H2 (2.5% hexane fraction), and H3 (5%
hexane fraction). All of these groups were added by
human calcium kidney stone and incubated at 37
o
C
for four hours. Calcium level was measured before
and after incubation by using atomic absorption
spectrophotometry method at 422.7 nm wavelenght.
2.5 Calcium Calibration Curve
Calcium calibration curve was prepared by using 6
different concentration which were 0 ppm, 0.2 ppm,
0.4 ppm, 0.6 ppm, 0.8 ppm and 1,0 ppm. All of these
concentrations will be measured by using atomic
absorption spectrophotometry method at 422.7 nm
wavelenght.
2.6 Calcium Determination in Sample
Before measurement of calcium level, the organic
compounds should be destructed by using nitric acid
65% which heated on a hot plate until the sample
solutin become transpicious. After that, the sample
solution measured using air-acetylene flame at
422.7 nm wavelenght.
3 RESULTS AND DISCUSSIONS
3.1 Calcium Calibration Curve
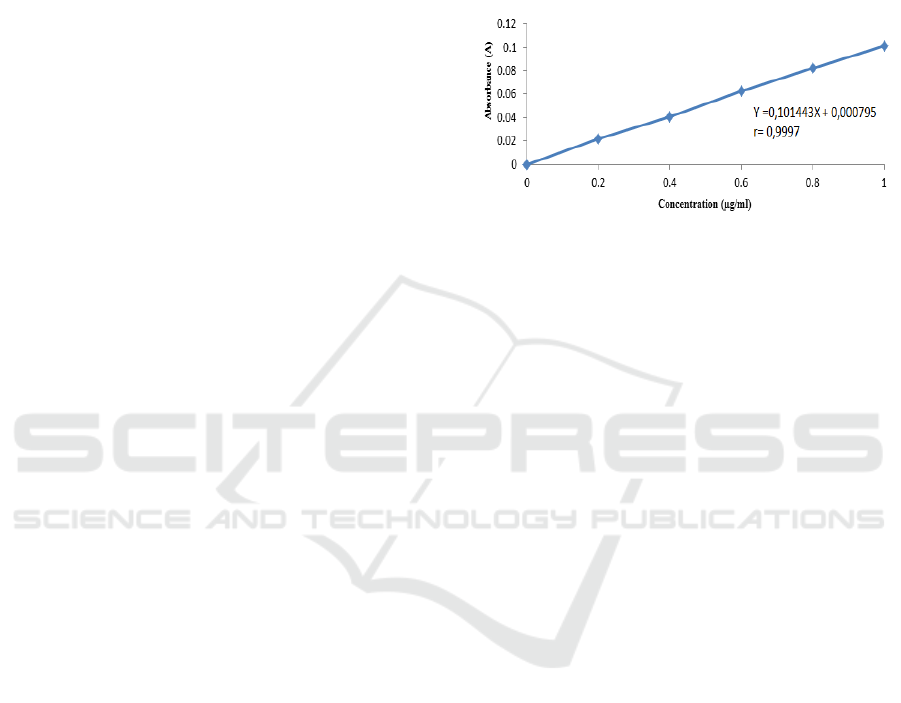
Calcium calibration curve is obtained by measuring
the absorbance of the standard solution at a
wavelength of 422.7 nm for calcium. Calibration
curve of calcium standard solution is as shown in the
figure below (Fig. 1). From the calculation results
obtained the correlation coefficient (r) of 0.9997 and
the regression line equation Y = 0.101443X +
0.000795. These results indicate that there is a linear
relationship between concentration and absorbance,
which is indicated by the value of the correlation
coefficient that is close to 1. The value of the
correlation coefficient obtained meets the
predetermined requirements, namely the value of the
correlation coefficient of not less than 0.995.
Figure 1: Calibration curve of calcium
3.2 The Effect of Chives Leaves Ethyl
Acetate and Hexane Fractions on
Solubility of Human Calcium
Kidney Stones
The level of of human calcium kidney stone
solubility is presented in Table 1. Based on the
results, it can be seen that ethyl acetate fraction
within dose 2.5% gave the highest activity in
dissolving the kidney stone campared to other
fraction. This can happen due to the potassium
which could happen because potassium is able to
push the calcium bond in kidney stone and can be
remove through urine (Putra et al., 2018; Iksen,
2015).
Other possibility is because of the phytochemical
compound especially flavonoid compounds which
are the main compound in ethyl acetate fraction. The
mechanism by flavonoid maybe caused by its
improving the dissolving effect by making a
complex bond with the calcium from kidney stone.
Calcium complex bond with flavonoid will be free
and can be remove through urine (Haro et al., 2017;
Sinaga et al., 2018).
4 CONCLUSIONS
Chives leaves ethyl acetate fraction solution with
2.5% concentration gives the highest activity in
dissolving calcium kidney stone. This can happen
due to the potassium and flavonoids compound from
chives leaves. It is hoped that this study could be an
alternative for the treatment of kidney stones
disease.
The Effect of Chives (Allium schoenoprasum L.) Leaves Ethyl Acetate and Hexane Fractions on Human Calcium Kidney Stones Solubility
by In Vitro Method
915
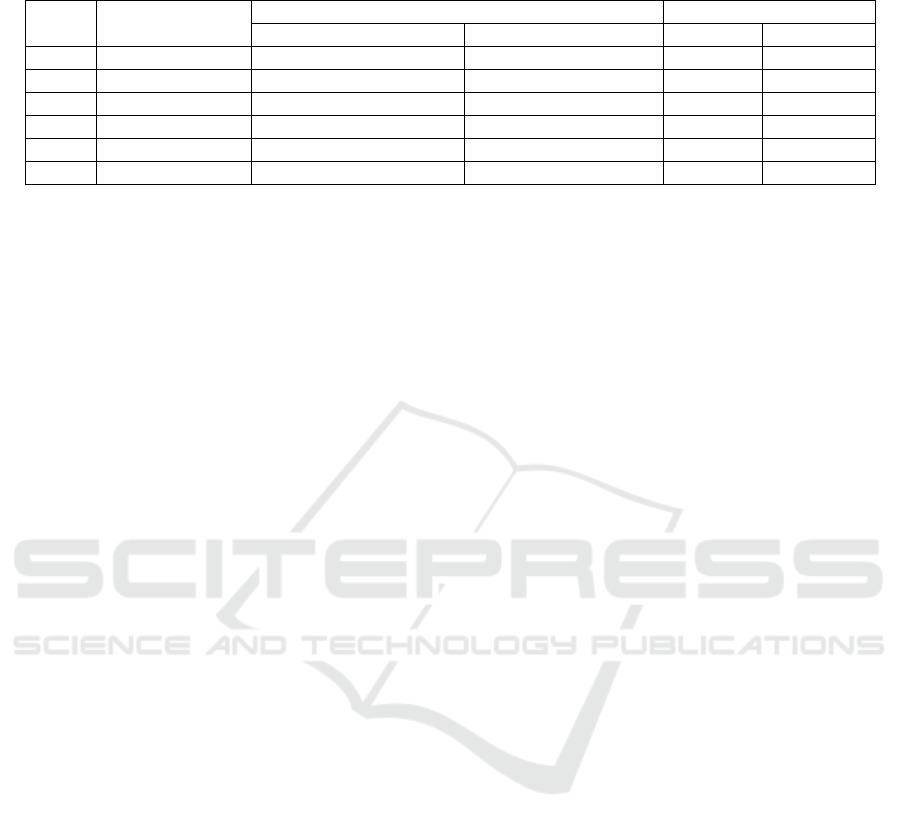
Table 1: The Effect of Chives Leaves Ethyl Acetate and Hexane Fractions on Solubility of Human Calcium Kidney Stones
No Treatments Calcium Level (μg/mL) Solubility
Before Incubation After Incubation (μg/mL) (%)
1 EA1 (1%) 1.81 20.79 18.98 91.30
2 EA2 (2.5%) 2.85 35.70 32.84 91.99
3 EA3 ( 5%) 3.74 29.97 26.22 87.49
4 H1 (1%) 11.78 13.80 2.02 14.63
5 H2 (2.5%) 13.71 16.17 2.45 15.15
6 H3 (5%) 20.88 23.91 3.03 12.67
ACKNOWLEDGEMENTS
The authors acknowledge the financial support by
Research Institutions, University of Sumatera Utara,
in accordance with the Contract Research
TALENTA (No: 2590/UN5.1.R/PPM/2018)
Universitas Sumatera Utara, Fiscal Year 2018.
Authors also thanks to Kevin and Marselina
Purnama Sari for their help for this research project.
REFERENCES
Al-Snafi, A. E. Pharmacological effects of allium species
grown in Iraq. (2014). International Journal of
Pharmaceuticals and Health Care Research, 1(4):
132-155.
American Urological Association (AUA). Medical
Management of Kidney Stones. AUA Guideline.
Ditjen POM., 1995. Indonesia Pharmacopoeia, Indonesia
Ministry of Health. Jakarta, 4
th
edition.
Depkes RI., 1995. Materia Medica, Indonesia Ministry of
Health. Jakarta, 6
th
edition.
Haro, G., Sinaga, S. M., Iksen, I., Nerdy, N., and
Theerachetmongkol, S. (2017). Protective effects of
chives leaves (Allium Schoenoprasum, L.) infusion
against ethylene glycol and ammonium chloride
induced nephrolithiasis in rats. Journal of Applied
Pharmaceutical Science, 7(8): 222-225.
Iksen, I., 2015. Determination of Calcium, Potassium and
Sodium Level in Fresh and Boild Chives Leaves
(Allium schoenoprasum L.) by Using Atomic
Absorption SpectrophotometryMethod, USU Press.
Medan.
Iksen, I., Haro, G., and Sinaga, S. M. (2017). In vitro test
of chive leaves infuse (Allium schoenoprasum, L.) on
calcium oxalate solubility using atomic absorption
spectrophotometry. International Journal of
ChemTech Research, 10(2): 99-102.
Mugni, A. I., 2013. Assay Activity of 70% Ethanol Extract
of Stem Bark of Kapok Randu to Inhibit The Forming
of a Kidney Stone on White Male Rats, UIN Syarif
Hidayatullah Press, Jakarta.
Putra, E. D. L., Ginting, N., Nazliniwaty, N., Iksen, I.,
Kurniawan, E., and Nerdy, N. (2018). In Vitro
antinehrolithiasis effect of breadfruit leaves extract by
atomic absorption spectrophotometry. Asian Journal
of Pharmaceutical and Clinical Research, 11(Special
Issue 1): 206-209.
Sinaga, S. M., Iksen, I., Haro, G., and Wardhany,
S.(2018). Potency of chives (Allium schoenoprasum,
L.) leaves infuse as inhibitor calcium lithogenesis on
urinary tract. Asian Journal of Pharmaceutical and
Clinical Research, 11(3): 77-80.
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
916
