
Effect of Lawsonia inermis Linnaeus Leaf Ethyl Acetate Extract on
Liver in Normal Rat
Tri Widyawati
1*
, Siti Syarifah
1
, Dwi Rita Anggraini
2
1
Department of Pharmacology and Therapeutic, Faculty of Medicine, Universitas Sumatera Utara,
Medan, 20155, Indonesia
2
Department of Anatomy, Faculty of Medicine, Universitas Sumatera Utara, Medan, 20155, Indonesia
Keywords: Lawsoniainermis, leaf, Ethyl Acetate, Extract, Liver
Abstract: Previous study revealed the antihyperglycemic activity and hepatoprotective effect of Lawsonia inermis
Linn. leaf extract (EAE) dose 1 g/kgbw in streptozotocin-induced diabetic rats. The present study was
conducted to evaluate the toxic effect of EAE on hepar in normal rat. EAE was obtained by serial extraction
using n-hexane and ethyl acetate (EAE). Two groups of Wistar rats (n=5) were treated with EAE (1.25
g/kgbw) and distilled water (NC: 10 ml/kgbw), orally, daily for 14 days, respectively. After 14 days the rats
were sacrified for histopathological evaluation of liver using hematoxyilline-eosin staining.The result
showed normal apperance of liver in NC-treated rat, contrarily in EAE-treated rats showed the hydrophic
degeneration, sinusoid and central vein congestion. Multiple nodules with strict lines, consisted of fat cells
undergoing proliferation, monocytes and limphocytes infiltration were also found. These results suggest that
EAE dose 1.25 g/kgbw toxic to liver and potentially destroy its function.
1 INTRODUCTION
Our previous study showed that ethyl acetate extract
of Lawsonia inermis Linnaues (EAE) leaf which
obtained by serial extraction (nhexane-ethylacetate-
ethanol-water) was the most active as
antihyperglycemic in streptozotocin-induced
diabetic rats at dose 1 g/kgbw. It was also
demonstrated the hepatoprotective effect of this
extract. Qualitative chemical screening of EAE
traced the presence of flavonoid, tannin, saponin and
glycoside (Widyawati et al, 2018). These chemical
compounds were suggested contributed to its benefit
pharmacological activities. In order for medicinal
plants to be utilized, it must be supported not only
the efficacy, but also its safety through toxicity test.
Liver is one of organ that have important role to
detoxify the xenobiotic including herbs (Gao et al,
2008; Nasri, 2013; Teschke, 2015). The purpose of
this study is to evaluate the effect of EAE at higher
dose 1.25 g/kgbw on liver in normal rat.
2 MATERIAL AND METHODS
2.1 Plant Material Collection and
Preparation of Ethyl Acetate
Extract
L. inermisLinn. leaves were collected from
TitiKuning, Medan, Indonesia (Coordinate:
3.526093, 98.684528). The plant was identified at
“Herbarium Bogoriense”, the Research Centre for
Biology-Indonesian Institute of Science, Bogor,
Indonesia and given a herbarium identification
number - No.924/IPH.1.01/If.07/III/2017. The fresh
leaves were dried under shade and ground into
powder. About 1.5 kg of the powdered leaf was
extracted serially by maceration in n-hexane and
ethyl acetate (EAE). The freeze-dried extracts were
kept in the freezer (-20C) before used.
2.2 Animals
Healthy male Wistar rats weighing between 180-250
g were obtained from animal house of Universitas
Sumatera Utara. The animals were acclimatized at
room temperature and a 12-h dark/light cycle, and
Widyawati, T., Syarifah, S. and Anggraini, D.
Effect of Lawsonia inermis Linnaeus Leaf Ethyl Acetate Extract on Liver in Normal Rat.
DOI: 10.5220/0010100909110913
In Proceedings of the International Conference of Science, Technology, Engineering, Environmental and Ramification Researches (ICOSTEERR 2018) - Research in Industry 4.0, pages
911-913
ISBN: 978-989-758-449-7
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
911
were allowed to access food and water ad libitum for
one week before being used for experimentation.The
study was performed after approved by Animal
Research Ethics Committees (AREEC), Faculty of
Mathematics and Natural Sciences (FMIPA),
Universitas Sumatera Utara.
2.3 Experimental Procedure
Rats were divided into two groups, n=5,
respectively. Group I (EAE-treated group) were
given EAE 1.25 g/kgbw, while group II were given
distilled water 10 ml/kgbw (NC-treated group). The
treatments were administered orally, at single dose
and followed for 14 days.
2.4 Preparation of Liver for
Histopathological Examination
The rats were sacrified with the carbogen gas (95%
O2 and 5% O2) and the liver was excised. The liver
was fixed in 10% buffered formaldehyde for 24
hours, followed by dehydrationusing 70% alcohol
(60 min), 96% alcohol (45 min), and absolute
alcohol (2 h). The clearing phase of the samples was
made by repeated xylene immersions, followed by
paraffin wax infiltrations.The samples were then
automatically processed with tissue processor
Thermo Scientific STP 120-3 and paraffin
embedding was prepared using modular tissue
embedding center Thermo Scientific Microm EC
350-1. The parafffin-embedded tissues were
sectioned into 5μm using the Leica RM 125RTS
microtome and mounted on a microscope slides. The
mounted slides were stained with hematoxylline (H)
and eosin (E) according to H&E staining technique.
The stained sections were then mounted in DPX
mounting medium with cover slide.
2.5 Histopathological Interpretation
Histopathological appearance of the liver was
evaluated by macroscopic and microscopic.
Degree of liver destruction were determined as
follow:
0 = normal or no destruction
+= one of these criteria was found; fatty
degeneration, or congestion of central vein and
sinusoid, focal necrosis, benign cysts with fat
++= two of these criteria were found; fatty
degeneration, or congestion of central vein and
sinusoid, focal necrosis, benign cysts with fat
+++= 3-4 criteria: fatty degeneration, or congestion
of central vein and sinusoid, focal necrosis, benign
cysts with fat
2.6 Photomicrography and Image
Analysis
Records of the histopathological results were
obtained by photomicrography using digital
photomicrographic microscope (Olympus BX 41
and Olympus DP25 video camera) at the
Anatomic Pathology Laboratory, Department of
Anatomic Pathology, Universitas Sumatera Utara.
3 RESULTS
Table 1 showed the degree of liver destruction in
NC- and EAE-treated rats. No destruction (Degree
0) were found in normal rat. Generally, EAE-treated
rats showed level 2 of liver destruction.
Table 1: Liver destruction degree macroscopically.
Grou
p
De
g
ree
NC 0
EAE1 +++
EAE2 ++
EAE3 +
EAE4 ++
EAE5 ++
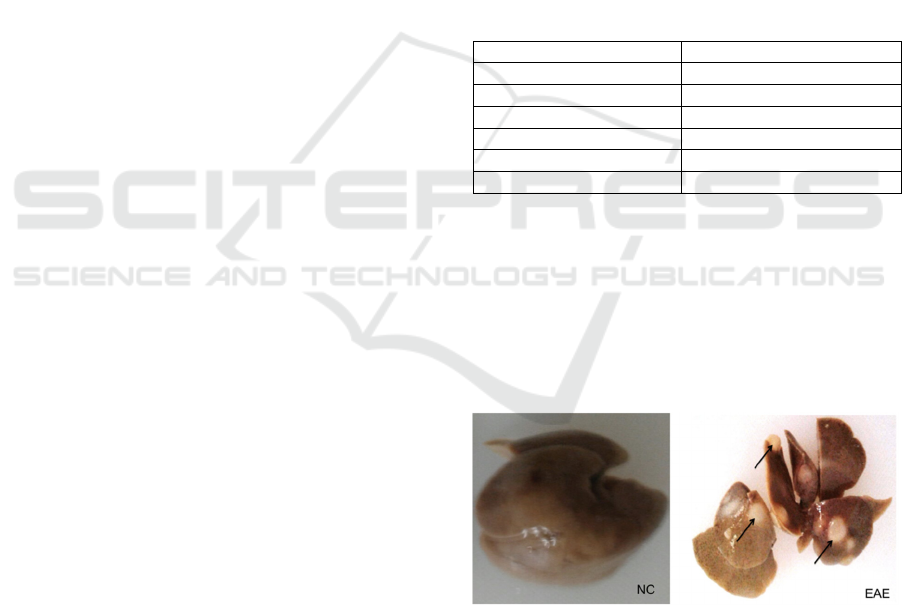
NC-treated group as control showed normal liver,
red to tan, well defined and soft consistency (Figure
1-NC), while grossly, the liver of EAE-treated group
showed pale tan to red, smooth and multiple cystic
(black arrow)appearance with diameter 0.2 cm. The
cysts relatively well-demarcated, white and smooth
(Figure 1-EAE).
Figure 1: Gross appearance of hepar NC- and EAE-
treated group (black arrow: cyst).
In normal hepatocyte (Figure 2-NC) showed normal
architecture with regular hepatocyte cells, round
nucleus, fine-chromatin cytoplasm eosinophilic;
normal sinusoid and central vein. Contrarily, the
liver cells of EAE-treated group showed congestion
in central vein and sinusoidal,enlargement
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
912
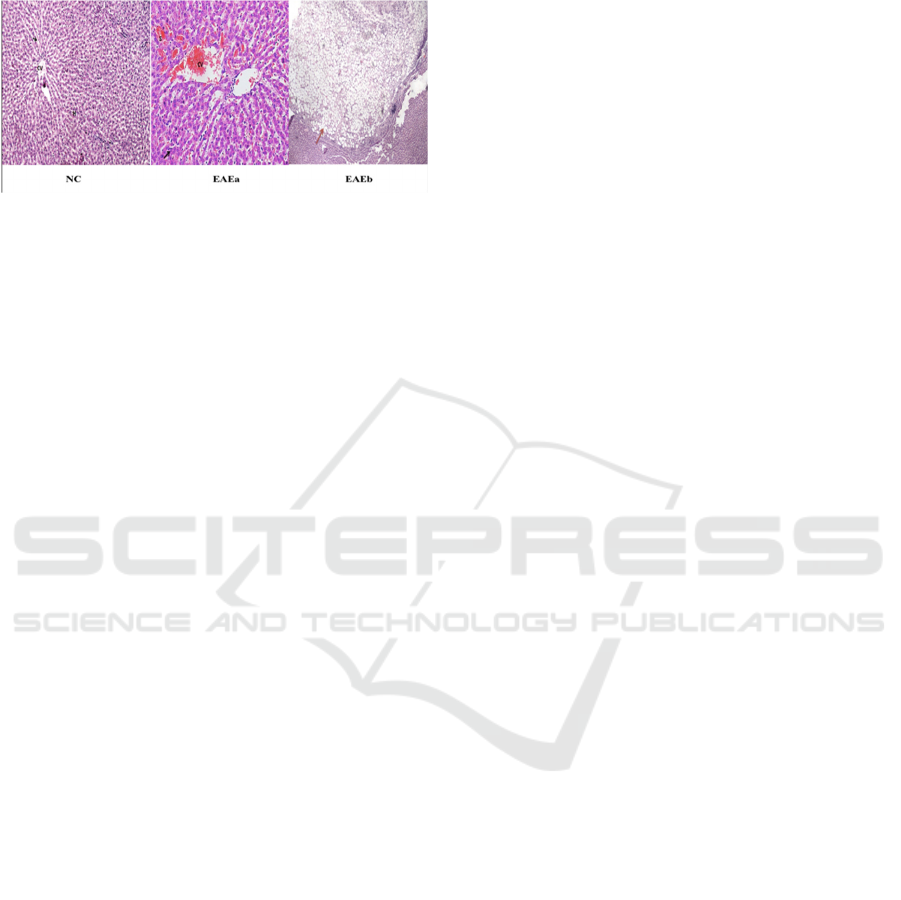
ofsinusoid, focal inflammation (black arrow) and
benign cysts contained proliferation of the fat cells
(red arrow) (Figure 2-EAEa-b).
Figure 2: The photomicrographs of liver section of NC-
and EAE-treated group (NC: 100x; EAE: 400x;
Hepatocyte cell (H); sinusoid (arrow); central vein (CV).
Herbal medicine derived from plant extracts are
being increasingly used to treat various of disease
(Seif, 2016). Some plant extracts and natural
compounds were found as hepatoprotective active
principles, while others adversely induced liver
toxicity (Manfo et al, 2016). The liver represents the
key "metabolic factory" is the most exposed organ
to xenobiotics including medicinal plant extracts.
This may be modulated by any compound
irrespective to the purpose of use. The
histopathological evaluation of the present study
showed that EAE dose 1.25 g/kgbw affected the
structure of liver. It was found clearly by the
changing of gross appearance of liver ie pale tan to
red and multiple cystic. This result contradictive
with our previous study that showed the
hepatoprotective effect of EAE dose 1 g/kgbw in
streptozotocin-induced diabetic rats. The higher dose
of EAE may have the role of this unwanted effect.
The action mechanisms involved in the
hepatoprotection or hepatotoxicity by the medicinal
plants are still not well elucidated.Herb induced liver
injury can be caused by the chemical compounds as
their causative agents. Elimination process for
metabolic degradation may yield hepatotoxic
metabolites that causing liver injury (Manfo et al,
2016; Frenzel and Teschke, 2016).
4 CONCLUSIONS
Ethyl acetate extract of Lawsonia inermis Linnaeus
leaf at dose 1.25 mg/kgbw toxic to the liver.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge to the
Universitas Sumatera Utara for supporting this study
(TALENTA USU GRANT 2018, No.
2590/UN5.1R/PPM/2018).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
Frenzel, C., Teschke, R., 2016.Herbal hepatotoxicity:
clinical characteristics and listing
compilation. International journal of molecular
sciences. 17(5), 588.
Gao, B., Jeong, W., I., Tian, Z., 2008.Liver: an organ
withpredominant innate immunity. Hepatology. 47(2),
729-736.
Manfo, F.,P.,T., Nantia, E.,A., Kuete, V.,
2014.Hepatotoxicity and hepatoprotective effects of
African medicinal plants. In Toxicological Survey of
African Medicinal Plants. 323-355.
Nasri, H., 2013.Toxicity and safety of medicinal
plants. Journal of HerbMed Pharmacology.2.
Seif, H., S., A., 2016.Physiological changes due
tohepatotoxicity and the protective role of some
medicinal plants. Beni-Suef University Journal of
Basic and Applied Sciences. 5(2), 134-146.
Teschke, R., Wolff, A., Frenzel, C., Eickhoff, A.,
Schulze,J., 2015.Herbal traditional Chinese medicine
and its evidence base in gastrointestinal
disorders. World J. Gastroenterol. 21:4466–4490.
Widyawati, T., Wahyuni, H.,S., Syarifah, S., Anggraini,
D.,R., Sari, M., I., 2018.Phytochemical Profile and
Antioxidant Assay of Ethyl Acetateof Lawsoniai
nermis (Linn) Leaf Extract. International Journal of
Chem Tech Research. 11(05), 78-84.
Effect of Lawsonia inermis Linnaeus Leaf Ethyl Acetate Extract on Liver in Normal Rat
913
