
A Survey on Aspergillus chevalieri Infection Isolated from Poultry
Feed
K. Nurtjahja
1*
, Yurnaliza
1
, A. Bungsu
1
, J. Simanullang
1
, J. E. Silalahi
1
, B. N. L. Gultom
1
, Sartini
2
1
Department of Biology, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Jln. Bioteknologi no.
1, Medan, North Sumatera, Indonesia 20155
2
Biology Faculty, Universitas Medan Area, Jln. Kolam no. 1 Medan, North Sumatera, Indonesia 20223
Keywords: Aspergillus chevalieri, Fungal Population, Poultry Feed.
Abstract: Population and characteristics Aspergillus chevalieri in finished poultry feed were investigated. The aim was
to isolate and enumerate Aspergillus chevalieri from finished poultry feed. Thirteen samples poultry feed of
chick starter, broiler and layer, sold by retailers in traditional markets were collected. Moisture content of the
feeds was determined by oven dried method. Fungal population was analyzed using dichloran 18% glycerol
agar (DG18) medium. All fungal colonies were isolated and incubated in potato dextrose agar slants (7 days,
29 °C). Morphological characteristics were observed. Results showed that chick starter has the highest
moisture content (10%) and the most infected by Aspergillus chevalieri (31.33×10
4
cfu g
-1
) followed by
broiler (0.33×10
3
cfu g
-1
) and none in layer.
1 INTRODUCTION
The occurence of fungal infection on poultry feed in
tropical countries become the primary cause
deterioration during storage. High rainfall and
relative humidity promote improper stored feed
absorb water favour from the environment. This
process leads to an increase moisture content exceeds
critical value for fungal growth and it may
contaminated by mycotoxins (JECFA, 1998). Maize,
soybean, rice bran, peanut and by product that major
of the component are susceptible infected by molds
(Okoly et al. 2007; Okun et al. 2015). Among fungal
genera, Aspergillus, Fusarium, Rhizopus, Penicillium
and Mucor were the most common found
contaminating in poultry feed (Krnjaja et al. 2008;
Kana et al. 2013; Nemati et al. 2014; Kehinde et al.
2014). Infection of the molds deteriorate feeds and
reduce its nutritional componds. As xerophilic fungi,
A. chevaliery is able to grow on low water activity.
The presence of A.chevalieri on poultry feed might
occur when the fungus colonize the raw materials
during harvesting, grow in it during postharvest
handlings such as drying, transportation and storage.
The objective of this research was designed to survey
Aspergillus chevalieri infection and isolated from
finished poultry feed (chick stater feed, broiler and
layer feed) sold by retailers in traditional markets.
2 MATERIALS AND METHODS
2.1 Sample Collection
A total of 13 composite samples of finished poultry
feed: chick starter, broiler and layer, were collected
(500 g each sample) during the dry season (months of
May and June 2018) from different retailers in
Medan, North Sumatera, Indonesia. Each sample was
packed separately in sterilized polythylene bag and
stored (-4 °C) in refrigerator for further use.
2.2 Fungal Population and
Identification
Population of A. chevalieri was determined by
dilution method in dichloran 18% glycerol agar
(DG18 medium) according to Pitt and Hocking
(2009). Twenty five gram of each sample in 500 mL
erlenmeyer was diluted in 250 mL sterilized distilled
water. The suspension was homogenized using shaker
(Gallenkamp SG92-02-311, England) 100 rpm for 2
minutes. Four dilutions, 10
-1
, 10
-2
, 10
-3
and 10
-4
were
1070
Nurtjahja, K., Yurnaliza, ., Bungsu, A., Simanullang, J., Silalahi, J., Gultom, B. and Sartini, .
A Survey on Aspergillus chevalieri Infection Isolated from Poultry Feed.
DOI: 10.5220/0010100010701072
In Proceedings of the International Conference of Science, Technology, Engineering, Environmental and Ramification Researches (ICOSTEERR 2018) - Research in Industry 4.0, pages
1070-1072
ISBN: 978-989-758-449-7
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
made. From the 10
-3
or 10
-4
dilution, 1 mL was
transfered to petridish (diameter 9 cm) and pour
plated in DG18 medium. Each sample was cultured
triplicates. The plates were incubated at at 29±2ºC for
6 days. All colonies were counted as colony forming
unit (cfu g
-1
) of the sample and identified according
to Pitt and Hocking (2009). Macroscopic and
microscopic identification were conducted under
Zeiss Prima Star 37081 light microscope, Gottingen,
Germany.
2.3 Determination of Moisture Content
For determination of moisture content, every 50 g of
sample was put on an aluminum foil dish and dried in
an oven at 130
o
C for 2 hours and reweighed (BSI,
1980). Three replicates were made for each sample.
3 RESULTS AND DISCUSSION
All finished poultry feed sold by retailers commonly
packaged in polypropylene bag and stored in open air.
Moisture content of the poultry feed are presented in
Figure 1.
Figure 1: Moisture content (% wet basis) of finished poultry
feed (chick starter, broiler and layer feed) collected from
retailers at traditional markets in Medan, North Sumatera.
Chick starter has the highest moisture content
(10%) followed by layer (8.5%) and broiler (7%).
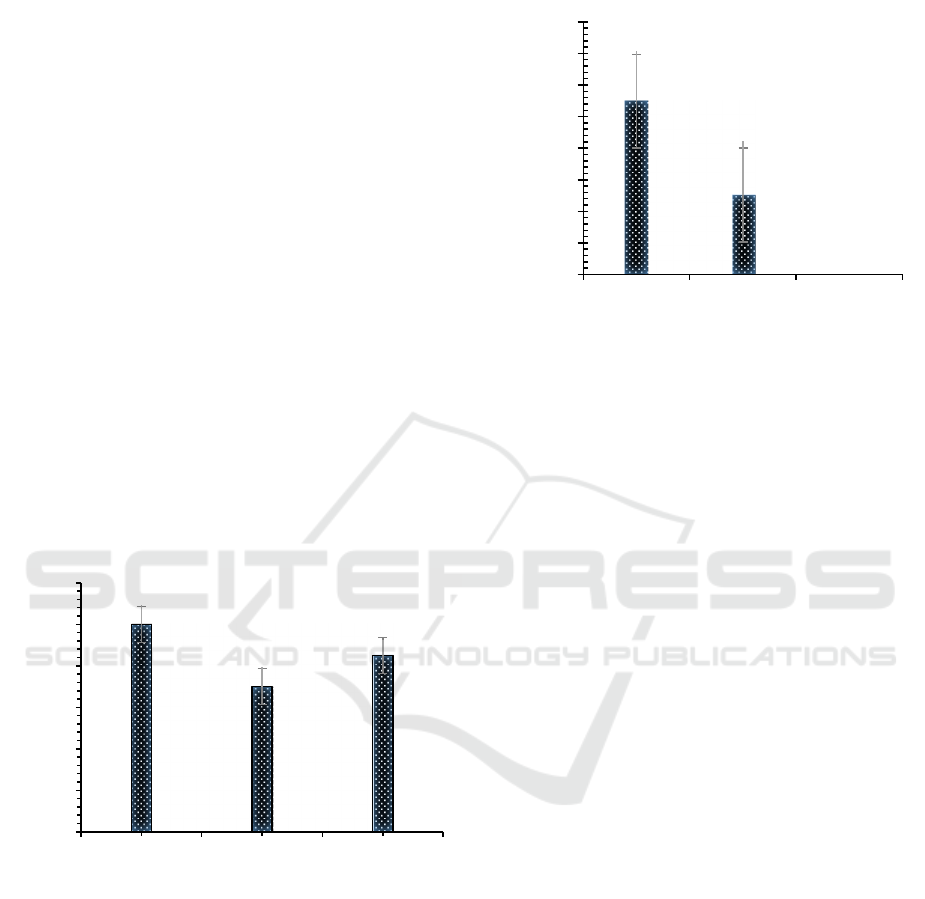
Feed moisture content determine A. chevalieri
population. Most of the colony was found in chick
starter (5.49 cfu g
-1
) followed by broiler (2.52 cfu g
-
1
). None A. chevalieri was found on layer (Figure 2).
Previous study by Saleemi et al. (2010) and
Sivakumar et al. (2014) reported that Aspergillus was
the most common on poultry feed. The most
prevalence of A. chevalieri on poultry feed was
reported by Greco et al. (2014).
Figure 2: Fungal population (cfu g
-1
) isolated from finished
poultry feed (chick starter, broiler and layer) after 6 days
incubation (29±2 °C) on dichloran 18% glycerol agar
(DG18) medium.
Poultry feed raw materials such as corn, rice bran,
peanut and soybean are vulnerable infected by
Aspergillus [Okun et al. 2015; Krnjaja et al. 2008).
We assumed the infection of the molds occurred on
poultry raw materials during harvest and their
population increase during storage.
4 CONCLUSION
All finished poultry feed sold by retailers in
traditional markets were infected by A. chevalieri.
The presence of the xerophilic mold potential
deterioration on poultry feed during storage.
Prevention fungal growth and deterioration were
required during storage of the feed.
ACKNOWLEDGEMENTS
The study was funded by Sumatera Utara University,
contract TALENTA Reseach no.
136/UN5.2.3.1/PPM/KP-TALENTA USU/2018.
REFERENCES
JECFA, (1998). Aflatoxins, Safety Evaluation of Certain
Food Additives and Contaminants. WHO
Okoly, IC. Ogbuewu, PI. Uchegbu, MC. Opara, MN.
Okorie, JO. Omede AA. Okoli, GC. Ibekwe, PI.
10
7
8,5
0
2
4
6
8
10
12
Chick starter Broiler Layer
Moisture content (% w.b)
Poultry feed
5,49
2,52
0
1
2
3
4
5
6
7
8
Chick starter Broiler layer
A. chevalieri population
(log cfu g
-1
)
Poultry feed
A Survey on Aspergillus chevalieri Infection Isolated from Poultry Feed
1071

(2007). Assessment of mycoflora of poutry feed raw
materials in humid tropical environment. J. American
Sci. 3: 5-9.
BSI, (1980). Methods of test for cereals and pulses. Part
3. Determination of moisture content of cereal and
cereal products (routine methods). British Standard
Institution. ISBN 0580 11 4333.
Greco, MV, Franci,ML, Golba, SLR. Pardo, AG. Pose,
GM. (2014). The Scientific World Journal, vol.4,
Article ID 968215,
http://dx.doi.org/10.1155/2014/968215.
Krnjaja, V, Stojanović, LJ. Cmiljanić, R. Trenkovski,
S. Tomašević, D. (2008). Fungal contamination and
natural occurrence of ochratoxin A (OTA) in poultry
feed. Biotechnol. in Animal Husbandry 24: 87-93.
Kana, JR. Gnonlonfin, BGJ. Harvey, J. Wainaina J. Wanjuki,
I., Skilton, RA. Teguia, A. (2013). Assessment of
aflatoxin contamination of maize, peanut meal, and
poltry feed mixtures from different agroecological zones
in Cameroon. J Animal and Poultry Sci. 2: 98-107.
Kehinde, MT. Oluwafemi, F. Itoandon, EE. Orji, FA. Ajayi,
OI. (2014). Fungal profile and aflatoxin contamination in
poultry feeds sold in Abeokuta, Ogun State, Nigeria. Nigerian
Food Journal 32: 73-79.
Nemati, Z. Janmohammadi, H. Taghijadeh, A. Nejad, HM.
Mogaddam, GH. Arzanlou, M. (2014). European J.
Zoological Res. 3: 56-60.
Okun, DO. Khamis, FM. Muluvi, GM. Ngeranwa, JJ.
Ombura, FO. Yongo, MO. Kenya, EU. (2015).
Distribution of indigenous strains of atoxigenic and
toxigenic Aspergillus flavus and Aspergillus
parasiticus in maize and peanuts agro-ecological zones
of Kenya. Agriculture and Food Security 14 2-10.
Pitt, JI. Hocking, AD. (2009). Fungi and Food Spoilage.
Springer, New York, 3rd edition.
Saleemi, MK. Khan, MZ. Khan, A. Javed, I. (2010).
Mycoflora of poultry feeds and mycotoxins producing
potential of Aspergillus species. Pak J Bot. 42: 427-
434.
Sivakumar, VK. Singaravelu, G. Sivamani, V. (2014).
Isolation, characterization and growth optimization of
toxigenic moulds from different animal feeds in
Tamilnadu. Int. J Curr. Microbiol. Appl. Sci. 3: 430-
445.
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
1072
