
Morphology of Composite Membranes based on Chitosan-Pahae
Natural Zeolite
Yuan Alfinsyah Sihombing
1,3
, Susilawati
1,3
, Irwana Nainggolan
2,3
, Adi Syahputra Purba
1
and Tulus
Ikhsan Nasution
1,3
1
Department of Physics, Faculty of Mathematics and Natural Science, Universitas Sumatera Utara, Medan, 20155,
Indonesia
2
Department of Chemistry, Faculty of Mathematics and Natural Science, Universitas Sumatera Utara, Medan, 20155,
Indonesia
3
Pusat Unggulan Inovasi Green Chitosan dan Material Maju, Universitas Sumatera Utara, Medan, 20155, Indonesia
Keywords: Chitosan, Pahae Natural Zeolite and solution casting method.
Abstract: Composite membranes based on Chitosan-Pahae Natural Zeolite have been fabricated by solution casting
method. The composite membranes are Chitosan and Chitosan with variation of composition zeolite relative
to the mass of chitosan. The composition of Pahae natural zeolite are 5%, 10%, 15%, 20% and 25%. The
samples were made by two step. The first step, the zeolite rock was crushed by mortar, and then zeolite was
sieved with particle size of 200 mesh. Zeolite was activated by soaking into Sulfuric acid for 2 hours, then
rinsed with distilled water until the pH about 7.0. Furthermore, the zeolite was sieved and burned in furnace
with temperature 100°C for 5 hours. The second step, chitosan was dissolved in the acetic acid solution and
stirred by using magnetic stirrer. Furthermore, zeolite was added into the solution. The resulting solution were
stirred for 24 hours and dried in atmosphere at room temperature. From FTIR spectra was confirmed the
existence of chitosan with stretching and bending at absorption wavenumber of 3250 cm
-1
, 2877 cm
-1
, 1640
cm
-1
, 1543 cm
-1
, 1401 cm
-1
and 1014 cm
-1
. The morphology of surfaces were obtained by using Scanning
Electron Microscope (SEM) and showed that zeolite was evenly distributed within chitosan.
1 INTRODUCTION
One of the polymer materials that is widely used in
making a membranes is chitosan. Chitosan is natural
polymers that has good characteristic such as
chemical inertness, hydrophilicity, biodegradability,
biocompatibility, good film formation properties and
more low cost (Liu et al, 2005 and Yavuz et al, 2009).
The membranes based on chitosan have received
considerable attention as membrane of polymer
electrolyte because the membranes have good
thermal and chemical stability, low conductivity,
good mechanical properties, etc. Low conductivity
properties caused by the absence of hydrogen ions
moving in the structure (Xiao et al, 2013). So, for
increase the efficient membranes based on chitosan,
various modification approaches are needed like
doping, blending and cross-linking.
The modification approaches based on chitosan
have been done by adding other materials such as
Carbon Nanotubes (CNTs), Polyaniline/Silica
(PAni/SiO
2
) and Sulfonated Graphene Oxide (SGO),
etc. CNTs were used to modify polymer electrolyte
membranes in energy conversion devices. The
addition of CNTs to chitosan matrix cause the
conductivity is increased (Wang et al, 2018). Ionic
cross-linked based on chitosan by using
nanocomposites of PAni/SiO
2
show increased
mechanical properties and improved the stability of
oxidation (Vijayalekshmi and Dipak, 2018).
Sulfonated chitosan (SCS) and SGO nanosheets are
fused into a membrane of chitosan have effect on the
electrochemical properties of the membrane such as
the increasing of conductivity, the reducing of
permeability and increasing of selectivity relative to
the pure chitosan. Furthermore, the addition of SCS
and SGO to chitosan leads more proton conductivity
than the individual additives due to the synergistic
effect of SCS and SGO (Shirdast et al, 2016).
Zeolite is alumino-silicate compound with
tetrahedral bound linked by oxygen. Aluminium
Atom is negative that can be neralized by cation. The
1052
Sihombing, Y., Susilawati, ., Nainggolan, I., Purba, A. and Nasution, T.
Morphology of Composite Membranes based on Chitosan-Pahae Natural Zeolite.
DOI: 10.5220/0010097010521056
In Proceedings of the International Conference of Science, Technology, Engineering, Environmental and Ramification Researches (ICOSTEERR 2018) - Research in Industry 4.0, pages
1052-1056
ISBN: 978-989-758-449-7
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

exchangeable cation affects the adsorption ability of
zeolite. For example, the zeolite was used as water
vapor filter to purify hydrogen gas (Susilawati et al,
2017). Besides that, Zeolite is inorganic materials that
has good mechanical properties and thermal stability.
It can be a great potential to modify chitosan. The
presence of hydrogen bonds between chitosan and
zeolite, the membranes shows the desired thermal and
mechanical stability (Wang et al, 2008).
In this study, chitosan was chosen as a matrix and
Pahae Natural zeolite as a filler. Pahae Natural Zeolite
was used because this mineral rock is widely
available in Indonesia, especially Tapanuli Utara,
Sumatera Utara. This study aims to fabricate
composite membranes and knowing the morphology
of membranes.
2 EXPERIMENTAL METHOD
2.1 Materials
There are two main materials in this study, Chitosan
and Zeolite. Chitosan medium molecular weight with
a degree of deacetylation about 85% was obtained
from Sigma Aldrich Chemical (Singapore) and
Natural Zeolite was obtained from Tarutung,
Tapanuli Utara, Sumatera Utara. Furthermore, some
of chemical materials needed to fabricate composite
membranes, such as acetic acid, sulfuric acid and
distilled water.
2.2 Membranes Preparation
The pure chitosan membrane and Chitosan-Zeolite
membranes were fabricated by using solution-casting
method. For the first, zeolite rock was crushed by
mortar and then this zeolite was sieved with particle
size of 200 mesh become zeolite powder. After that,
zeolite powder was activated by soaking into Sulfuric
acid 6% for 2 hours using magnetic stirrer and hot
plate. Then the zeolite powder was flushed with
distilled water until the pH of flushing solution is
reached normal pH about 7.0, which confirmed that
zeolite powder was completely free of sulfuric acid.
Furthermore, the zeolite powder was sieved by sieve
paper and then burned in furnace with temperature
100°C for 5 hours.
The second step, 1.5 g of chitosan was dissolved
in the 75 ml, 2wt% acetic acid solution. Then, this
solution of chitosan and acetic acid were stirred by
using magnetic stirrer and hot plate. After chitosan
and acetic acid were mixed, the zeolite powder was
added into this solution with variation zeolite com-
position of 5%, 10%, 15%, 20% and 25%. The resul-
ting solution were stirred for 24 hours and then this
mixtures were poured onto a glass mold and dried in
atmosphere pressure at room temperature. Finally, the
composite membranes were obtained. For pure chi-
tosan membrane, the chitosan was fabricated in same
way with others devoid adding of zeolite powder.
2.3 Characterization
2.3.1 Spectra of Fourier Transform Infrared
(FTIR)
The spectra of Fourier Transform Infrared were
measured by Agilent/Cary 630 in transmittance
mode. This instrument has resolution of 16 cm
-1
and
spectra of every sample was measured in the
wavenumber range between 4050 cm
-1
and 650 cm
-1
at room temperature.
2.3.2 Scanning Electron Microscope (SEM)
For scan of the surface morphology of composite
membranes were used by Zeiss/SEM EVO MA10
instrument with magnification 500 x. The SEM
morphology was obtained to show the existence of
zeolite in composite membranes.
3 RESULTS AND DISCUSSION
3.1 Spectra of Fourier Transform
Infrared (FTIR)
The spectra of FTIR ensured the existence of
hydrogen bonds. The hydrogen bonds occur between
chitosan and Pahae Natural zeolite in composite
membranes. Figure 1 showed the FTIR spectra of
chitosan and composite membranes with variation of
zeolite composition. The peak of absorption spectra
for all of membranes at around 3250 cm
-1
. This
wavenumber was confirmed to stretching of hydroxyl
groups (-OH).
Aliphatic groups (CH
2
and –CH
3
) could be
observed about 2877 cm
-1
. The absorption peaks
around 1640 and 1543 cm
-1
were confirmed to C=O
stretching (the band of Amide I) and –NH
2
Bending
(the band of Amide II), respectively (Yuan et al,
2007). The existence of C-O Stretching of Primary
alcohol was showed by absorption of wavenumber at
1401 cm
-1
and the last of absorption peak was showed
at 1014 cm
-1
. It was confirmed to glycosidic –C-O-C-
groups that connect between Monomer of Chitosan.
Morphology of Composite Membranes based on Chitosan-Pahae Natural Zeolite
1053
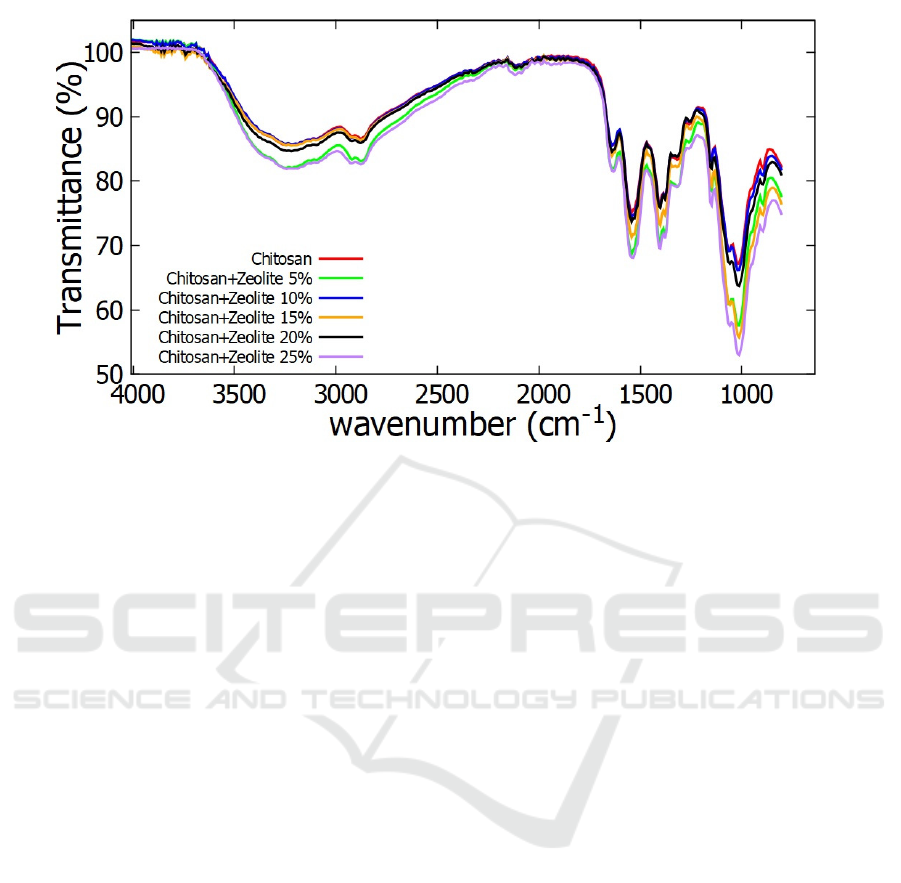
Figure 1: FTIR Spectra of Chitosan and composite membranes with variation of zeolite composition.
3.2 Scanning Electron Microscope
(SEM)
Morphological analysis in this research was
performed by using Scanning Electron Microscope
(SEM) with magnification 500 x. The surface of
membranes were scan by Zeiss/SEM. These surface
morphology could be seen from Figure 2 (a-f).
According to Figure 2, Pahae natural zeolite particles
distribution were relatively homogenous in the
chitosan phase because of its efficient dispersion.
Figure 2 also was confirmed that the increasing of
zeolite particles composition cause zeolite particles
more evenly distributed in chitosan. The surface of all
composite membranes were uniform and smooth
without tolerable defect. Finally, the composite
membranes from chitosan and zeolite was
successfully fabricated with no visible zeolite
aggregation existing in the membranes.
4 CONCLUSIONS
Composite membranes based on chitosan-zeolite
with variation of zeolite composition were obtained.
The variation of zeolite composition are 5%, 10%,
15%, 20% and 25% relative to mass of chitosan. From
FTIR spectra, the absorption peaks were occur at
wavenumber around 3250 cm
-1
, 2877 cm
-1
, 1640 cm
-
1
, 1543 cm
-1
, 1401 cm
-1
and 1014 cm
-1
. These
wavenumbers were confirmed to stretching of
hydroxyl groups, aliphatic groups. Amide I and II
band, C-O Stretching of Primary alcohol and
glycosidic –C-O-C- groups. All of this peaks related
to chitosan. Furthermore, the Pahae natural zeolite
could be evenly distributed in chitosan. It was
confirmed by scanning electron microscope
morphology. The surface of composite membranes
showed that membranes were uniform and smooth
without defect and aggregation.
ACKNOWLEDGEMENTS
The authors are very grateful to Universitas Sumatera
Utara for its funding according to TALENTA
research contract 2018 with number
2590/UN5.1.R/PPM/2017 on March 16
th
, 2018.
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
1054
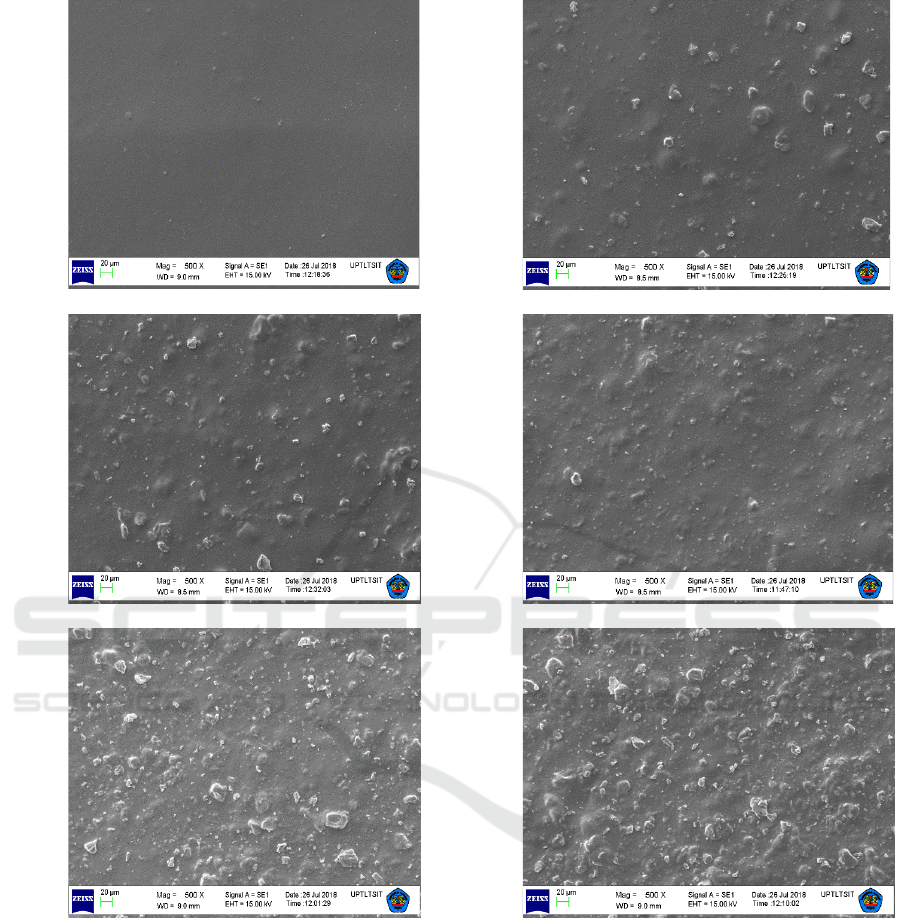
(a) (b)
(c) (d)
(e) (f)
Figure 2: Morphology of composite membrane by using SEM; (a) pure Chitosan, (b) Chitosan/Zeolite 5%, (c)
Chitosan/Zeolite 10%, (d) Chitosan/Zeolite 15%, (e) Chitosan/Zeolite 20% and (f) Chitosan/Zeolite 25%.
REFERENCES
Liu Y. L., Hsu C. Y., Su Y. H., and Lai J. Y. 2005.
Chitosan-silica complex membranes from sulfonic acid
functionalized silica nanoparticles for pervaporation
dehydration of ethanol-water solutions.
Biomacromolecules. 6 (1), 368-373.
Shirdast, A., Alireza S., and Mahdi A. 2016. Effect of
incorporation of sulfonated chitosan/sulfonated
graphene oxide on the proton conductivity of chitosan
membranes. Journal of Power Sources, 306, 541-551.
Susilawati, Tulus I. N., Fynnisa Z., and Hamonangan N.
2017. Hydrogen purification using natural pahae zeolit
and cocoa rind based filter. International Journal of
Applied Engineering Research 12 (13), 3914-3918.
Morphology of Composite Membranes based on Chitosan-Pahae Natural Zeolite
1055

Vijayalekshmi, V., and Dipak Khastgir. 2018. Hybrid
composite membranes of chitosan/sulfonated
polyaniline/silica as polymer electrolyte membrane for
fuel cells. Carbohydrate Polymers, 179, 152-163.
Wang, Jie, Chunli Gong, Sheng Wen, Hai Liu, Caiqin Qin,
Chuanxi Xiong and Lijie Dong. 2018. Proton exchange
membrane based on chitosan and solvent-free carbon
nanotube fluids for fuel cells applications.
Carbohydrate Polymers, 186, 200-207.
Wang, J., Xiao hang Z., Hong W., Bin Z., Zhongyi J.,
Xiaopeng H., and Baoyi W. 2008. Effect of zeolites on
chitosan/zeolite hybrid membranes for direct methanol
fuel cell. Journal of Power Sources 178, 9-19. Xiao Y.,
Xiang Y., Xiu R., and Lu S. 2013. Development of
cesium phosphotungstate salt and chitosan composite
membrane for direct methanol fuel cells. Carbohydrate
Polymers, 98 (1), 233–240.
Yavuz A. G., Uygun A., and Bhethanabotla V. R. 2009.
Substituted polyaniline/chitosan composites: synthesis
and characterization. Carbohydrate Polymers, 75(3),
448–453.
Yuan, W., Hong Wu, Bin Zheng, Xiaohong Zheng,
Zhongyi Jiang, Xiaopeng Hao, and Baoyi Wang. 2007.
Sorbitol-plasticized chitosan/zeolite hybrid membrane
for direct methanol fuel cell. Journal of Power Sources
172, 604-612
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
1056
