
The Preparation of Agar-chitosan Film from Gracilaria
M. Zulham Efendi Sinaga
1,3
, Saharman Gea
1
, Yuan Alfinsyah Sihombing
2,3
, Emma Zaidar
1,3
,
Rumondang Bulan
1
, Nami Panindia
1
, Hamdan Azhari
1
1
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Medan, 20155,
Indonesia.
2
Department of Physics, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Medan, 20155,
Indonesia.
3
Pusat Unggulan Inovasi (PUI) Green Chitosan dan Material Maju Universitas Sumatera, Indonesia.
ema3@usu.ac.idrumondang@usu.ac.idnamip14@gmail.com, and hamdanazhari91@gmail.com
Keywords: Chitosan, Agar, Gracilaria, Edible film, Food packaging.
Abstract: Packaging is one of the most important parts in maintaining the quality of food product. The film of agar-
glycerol-chitosan has been prepared fromthe isolated agar of Gracilaria. The objective of this study is to
investigate mechanical properties including the tensile strength, the young’s modulus, the elongation at
break,the functional groups by FTIR and the water solubility. The film was prepared by mixing agar, glycerol
(20 %) and chitosan (0 %, 1 %, 2 %, 3 % and 4 %) at 60
0
C and 600 rpm. FTIR showed that the interaction
occurred in the film agar-glycerol-chitosan was hydrogen bonding. From the result, the more chitosan added
to the agar-glycerol-chitosan film, the higher the tensile strength, the elongation at break, the young’s modulus
and the water solubulity.
1 INTRODUCTION
Packaging is one of the most important parts in
maintaining the quality of food products. The most
commonly used packaging is plastic, but the use of
plastic causes some problems such as difficult to
degradable in nature and environmentally unfriendly.
Similarly, there is a concern using plastic as
packaging which transfers plastic molecules to food.
Hence, edible film is considered as one of alternatives
for packaging food because it is environmentally
friendly and safe for consumption.
Some biopolymers used as edible film and coating
materials are polysaccharides, proteins and lipids
(Susanne and Gauri 2002; Paula et al., 2014; Kwang
et al., 2004). The biopolymers are available
abundantly in nature and they can be easily and
continuously obtained. Seaweed is one of potential
resources of polysaccharides. For example alginate,
agar, carrageenan, and vulvan. Agar is a derivative of
the polysaccharide present in the cell wall red algae
of Rhodophyceae class (Rafael,1995)which is widely
cultivated in Indonesia.
Chitin is the main componen of the exoskeleton
of crustacea like crabs and shrimps.Chitin is
composed of a monomer unit N-asetil-D-
glucosamine(2-acetamido-2-deoxy-Dglucopyranose)
linear bonding β-(1→4) (Maher and Entsar, 2013).
Chitin is white, hard, inelastic polysaccharide
whichhas much nitrogen, and it is the main resources
of pollutan in beach area.Chitosan is a derivative of
chitin and it is the second most abundant
polysaccharide after cellulose. Chitosan is a natural
polysaccharide resulting from the deacetylation
process (COCH
3
removal) of chitin. Generally,
chitosan is added to a film to produce excellent
biocompatibility film, forming ability and
antimicrobial activity. The other advantage of adding
chitosan is to decrease water sensitivity, increase
mechanical and barrier of film (Lili et al, 2017).
2 EXPERIMENTAL METHOD
2.1 Isolated Agar
Agar was prepared by soaking Gracilaria overnight
in a 200 ml NaOH 3%. After that, it was heated in a
water bath for 3 hours at 50 ºC and then washed with
deionized water. The process was followed by
Sinaga, M., Gea, S., Sihombing, Y., Zaidar, E., Bulan, R., Panindia, N. and Azhari, H.
The Preparation of Agar-chitosan Film from Gracilar ia.
DOI: 10.5220/0010096910471051
In Proceedings of the International Conference of Science, Technology, Engineering, Environmental and Ramification Researches (ICOSTEERR 2018) - Research in Industry 4.0, pages
1047-1051
ISBN: 978-989-758-449-7
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
1047

soaking Gracilaria twice in a H
2
SO
4
0.025 % and
deionised water for 2 hours. After that it was washed
with 300 ml deionized water for 1.5 hours until pH 6-
6,5. It was frozen, melted and dried for 24 hours at 60
ºC. Agar yield was determined as the percentage of
dry weight by the following equation:
Agar yield =
𝑥 100 % (1)
2.2 Film Preparation
Chitosan powder (1,2,3 and 4 g) was dissolved in a
100 ml acetic acid 0.2 M solution at 60 ºC and 600
rpm to produce chitosan solution. Agar powder (5
g) was dissolved in a 100 ml deionized water at 95 ºC
and 600 rpm for 60 minutes. An amount of 20% of
glycerol was added to the agar and chitosan solution
as plasticizer. The agar-chitosan film was fabricated
by mixing chitosan solution (1,2,3 and 4 g /
100 ml) and agar solution (5 g/100 ml) with a ratio of
1:1. The mixture was stirred at 60 ºC and 600 rpm for
60 minutes. An amount of
film solution was distributed into the template for
drying and casting for 24 hours at 40 ºC. The films
were stored in a desiccator.
2.3 Characterization
2.3.1 Spectra of Fourier Transform Infrared
(FTIR)
Agar-chitosan films were measured by using an
IRPrestige-21 Shimadzu - Japan to investigate the
interactions of agar, glycerol and chitosan films. The
sample was prepared by mixing the film and KBr with
ratio of 100 : 1. The FTIR was operated with a
wavelength of 400 – 4000 cm
-1
, a resolution of 4 cm
-
1
and 100 scans.
2.3.2 Mechanical Properties
The tensile strength, the elongation at break and the
young’s modulus of the films (100 mm x 25 mm)
weredetermined using a RTF-1350 Tensilon- Japan.
2.3.3 Water Solubility (WS)
The solubility of films in water (WS) is defined as
the percentage of dry matter dissolved in water from
the film dissolved after soaking in distilled
water. The films were cut into a size of 25 mm ×
20 mm and stored in a desiccator to obtain a constant
weight. The specimen was weighed to determine the
initial dry weight and placed in a glass containing a
50 mL of deionized water. The sample
was maintained with a constant stirring for 2 hours,
a temperature of 80 ºC and dried at 105 ºC until
a constant weight was obtained. The percentage of
total dissolved material is calculated as follows:
WS=
𝑥 100 %(2)
3 RESULTS AND DISCUSSION
The agar yield from this research was 48 %. There are
some factors that influence the isolation of Gracilaria
including soaking time, temperature, extraction time
and temperature (Mahdieh et al., 2013).With
reference to Figure 1, the film was initially dark
brown. It becomes brighter after the addition of
glycerol 20% and chitosan 1, 2, 3 and 4%.
3.1 FTIR Spectroscopic Analysis of
Film
FTIR spectral analysis of the agar film was performed
to identify the nature of the functional groups present.
From Figure 2, the peak at at 3556 cm
-1
is attributed to
OH stretching from hidroxyl groups (Babak and
Fatemeh, 2012). The peak at 1184 cm
-1
is attributed
to stretching vibration C-O in C-O-H groups. The
absorption at 1130 cm
-1
represents stretching
vibration C-O in C-O-C groups (Mayur and Sonal,
2010). In the chitosan spectrum the peak at 3371 cm
-
1
isassociated withstretching vibration N-H bending
and hydrogen bonded hidroxyl groups
(Lili et al,
2017).The peak at 1643 cm
-1
and 1600 cm
-1
representto amide II (N-H) and amide I (C=O)
respectively (Lagaron et al.,2007).The wavenumber
of the FTIR spectral of the film agar changed after the
addition of 20% glycerol. It can be seen from 3603
cm
-1
to 3317 cm
-1
for OH stretching of the hydroxyl
group , 1184 cm
-1
to 1107 cm
-1
for C-O of C-OH
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
1048

Figure 1 The Specimen of (a) Agar film, (b) agar-glycerol 20%, (c) agar-glycerol 20% – Chitosan1%, (d) agar-glycerol
20% - chitosan 2%, (e) agar-glycerol – chitosan 3% and (f) agar-glycerol – chitosan 4%.
1130 cm
-1
to 1045 cm
-1
for C-O in C-O-C. A new peak
was not appeared in the spectrum which indicated that
there was only hydrogen bonding between agar and
glycerol.Similarly, there is a shift of wavenumber
with the addition of 20% glycerol and chitosan
4%(Laura et al, 2016). They are from 3603 cm
-1
to
3317 cm
-1
indicating OH stretching of the hydroxyl
group, 1184 cm
-1
to 1107 cm
-1
for C-O of C-OH, 1130
cm
-1
to 1045 cm
-1
for C-O in C-O-C.There was
interaction between the functional group of O-H from
polysaccharides and N-H from chitosan (Emma et al,
2016).The agar film with glycerol 20 % and chitosan
4 % its known that the FTIR results show a
combination of the three constituent components
without forming a new functional groups. the
stretching OH of the hydroxyl group in film (3359
cm
-1
) was higher than agar (3556 cm
-1
) as well as
chitosan (3371 cm
-1
). This is due to inter and intra
molecular hydrogen bonding interactions of all
components
(Lili et al, 2017).
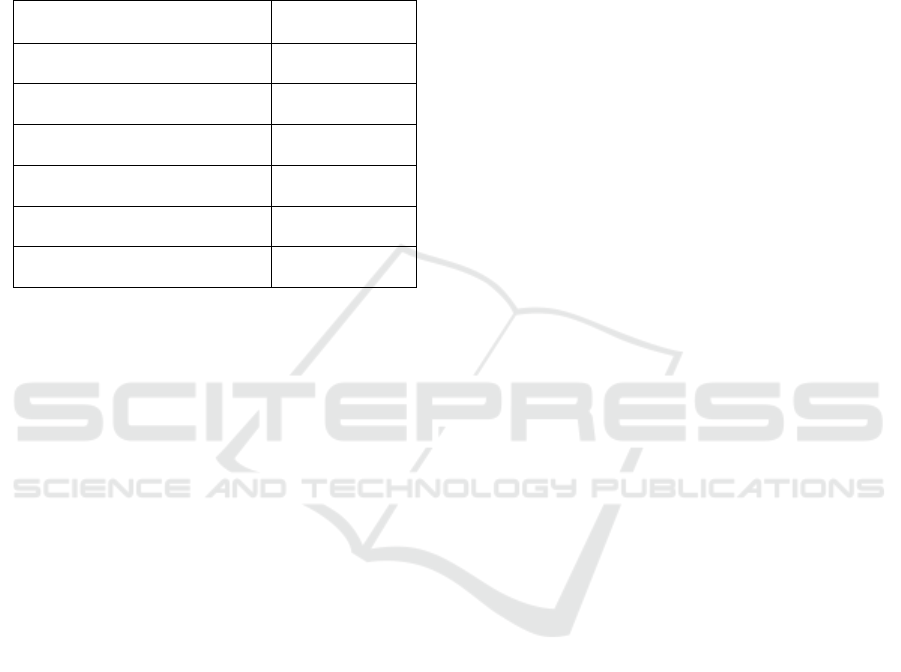
3.2 Mechanical Properties
The mechanical properties of edible film from this
research are show in Table 1.
Based on Table 1. film agar had the best mechanical
properties with the tensile strength of 3.785 MPa, but
the elongation at break was the lowest (0.199 %).
Table 1: Mechanical Properties of film agar, agar-glycerol
agar-glycerol-chitosan
Sample
Tensile
Strength
(
MPa
)
Young’s
Modulus
(
MPa
)
Elongation
atBreaks
(
%
)
Agar 3.785 1,993.31 0.199
Agar-glycerol
(20 %)
0.029 4.173 9.805
Agar-glycerol
(20%)-chitosan
(
1%
)
0.184 2.927 8.449
Agar-glycerol
(20%)-chitosan
(
2%
)
0.276 22.743 12.792
Agar-glycerol
(20%)-chitosan
(
3%
)
0.413 38.946 16.857
Agar-glycerol
(20%)-chitosan
(4%)
0.759 48.836 19.168
The addition of glycerol decreased the tensile strength
of film from 3.785 MPa to 0.029 MPa, increased the
value of elongation at break from 0.199 % to 9.805
%. The tensile strength and the elongation at break of
film increased after the addition of chitosan. The best
mechanical properties of the edible film were found
for agar-glycerol 20%-chitosan 4%.
a
b
c d
e
f
The Preparation of Agar-chitosan Film from Gracilaria
1049
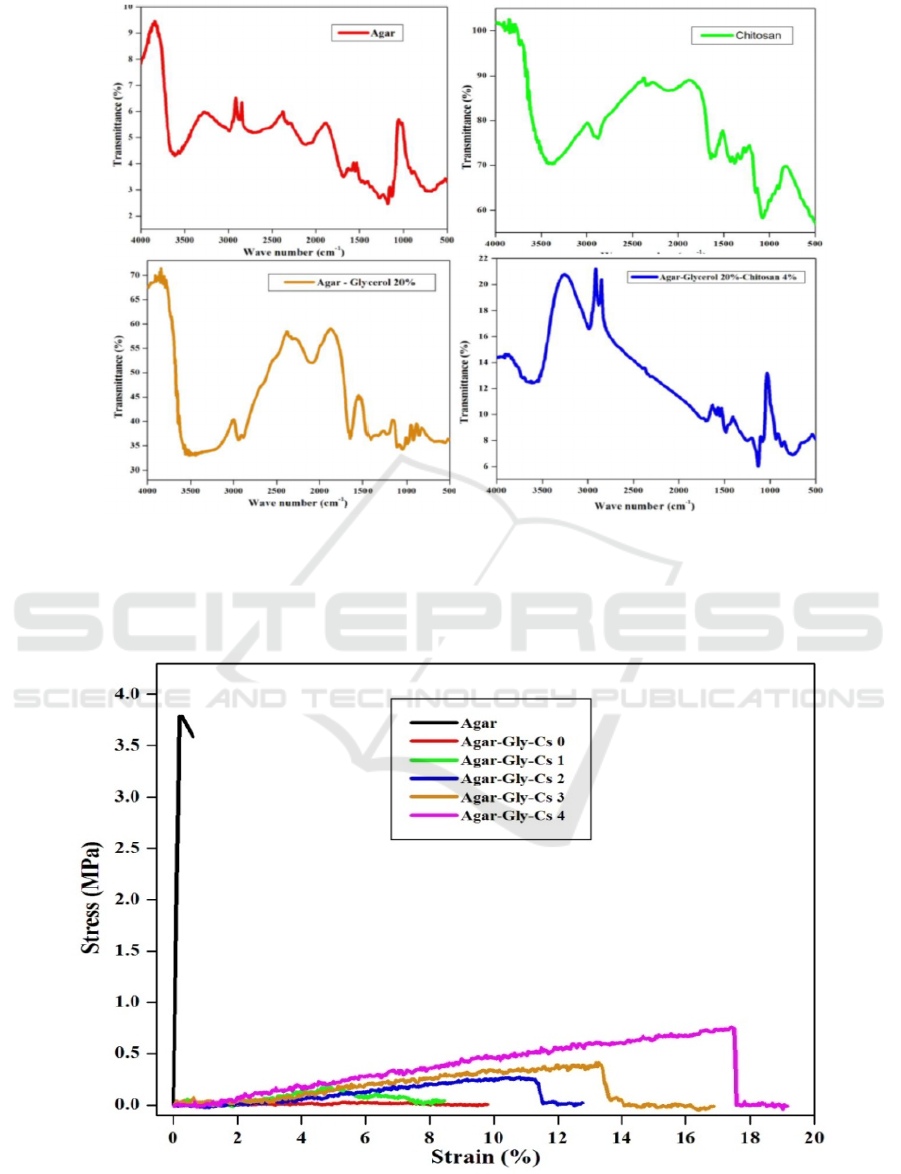
Figure 2: FTIR spectra of agar, chitosan, agar-glycerol 20 % and agar-glycerol 20 %-chitosan 4 %
Figure 3:The tensile strength of agar, agar-glycerol 20 %, agar-glycerol 20 %-chitosan 4 %
ICOSTEERR 2018 - International Conference of Science, Technology, Engineering, Environmental and Ramification Researches
1050
3.3 Water Solubility (WS)
Based on Table 2,the solubility of film increased by
the addition of chitosan. This is due to chitosan highly
soluble in water. However, agar forms gel in water.
Table 2: Water solubility (WS) of agar, agar-glycerol, agar-
glycerol-chitosan
Sample
Water Solubility
(
%
)
Agar 9.84
Agar-glycerol 20 % 88.19
Agar-glycerol 20%-chitosan 1% 69.57
Agar-glycerol 20%-chitosan 2% 80.02
Agar-glycerol 20%-chitosan 3% 85.58
Agar-glycerol 20%-chitosan 4% 87.78
For food packaging application, low solubility
filmisneeded in order to maintain the integrity of
structures to cover vegetables andfruits. However,
high film solubility can be preferred for films to wrap
candy
(Lili et al, 2017)
4 CONCLUSIONS
In summary, it has been shown from this reseach that
the result from the isolation of Gracilaria is 48%.
FTIR spectral indicates that there is hydrogen
bonding in the film agar-glycerol-chitosan. The more
the concentration of chitosan added to the filmagar-
glycerol-chitosan, the tensile strength, the elongation
at break, the young's modulus and the water solubility
increase.
ACKNOWLEDGEMENTS
The authors thank to the Rector of Universitas
Sumatera Utara for the financial support towards this
research in the TALENTA Project 2018,
No.2590/UN5.1.R/PPM/2017 on March.16. 2018.
REFERENCES
Babak S., Fatemeh A., 2012.Adsorptive removal of
methylene blue by agar: effects of NaCl and
ethanol. Chemistry Central Journal (6), 1-13.
Emma Z., Rumondang B., Zul A., Jimmy, M. S. S.,
2016. Modification of Mango Extract with
Mixture of Chitosan, Glycerine and Tapioca to
Produce Edible Film. Chemistry and Materials
Research 8(6), 25-30.
Kwang Y. L., Jaeyong S., Hyeon G. L.,
2004.Mechanical Properties Of Gellan and
Gelatin CompositeFilms. Carbohydrate
Polymers56(2),251-254.
Lagaron,J. M., Fernandez-Saiz, P., Ocio, M. J., 2007.
Using ATR-FTIR spectroscopyto design active
antimicrobial food packaging structures based on
high molecular weight chitosan polysaccharide.
Journal of Agricultural and Food Chemistry55(7),
2554-2562.
Laura F. C., Marian M., Angela S., Leira A., M.,
Carmen V., Elena D. A., Angeles H., 2016. Films
Of Chitosan And Chitosan-Oligosaccharide
Neutralized And Thermally Treated: Effects on Its
Antibacterial and Other Activities. LWT - Food
Science and Technology73, 368-374.
Lili R., Xiaoxia Y., Jiang Z., Jin T., Xingguang S.,
2017. Influence of Chitosan Concentration On
Mechanical and Barrier Properties of Corn
Starch/Chitosan Films. International Journal of
Biological Macromolecules105, 1636–1643.
Mahdieh K. Y., Houman R. I., Yousef F., 2013.
Effect of Extraction Process on Agar Properties of
Gracilaria corticata (Rhodophyta) Collected from
the Persian Golf. Phycologia52(5), 1-9.
Maher Z. E., Entsar S. A., 2013. Chitosan Based
Edible Films And Coatings: A review. Materials
Science and Engineering C33, 1819–1841.
Mayur V.,Sonal T., 2010. Isocyanate Crosslinked
Reactive Starch Nanoparticles for Thermo-
ResponsiveConducting Applications,
Carbohydr345, 2354–2360.
Paula J. P. E., Wen-Xian D., Roberto J. A. B.,Nilda
F. F. S., Tara H.M., 2014. Edible Films From
Pectin: Physical-Mechanical and Antimicrobial
Properties - A Review. Food Hydrocolloids35,
287-296.
Rafael A.,1995. Word-wide Use and Importance of
Gracilaria.J. Appl. Phycol7, 231-243.
Susanne A., Gauri S. M., 2002. Comparative
Evaluation of EdibleCoatings to Reduce Fat
Uptake in a Deep-Fried Cereal Product. Food
Research International35, 445-458.
The Preparation of Agar-chitosan Film from Gracilaria
1051
